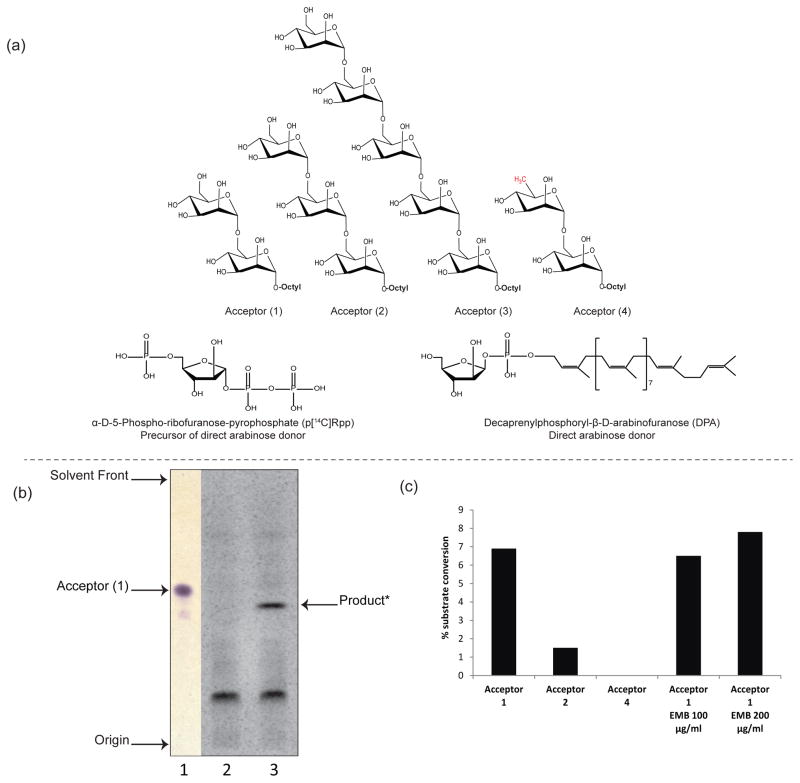
Figure 2. Evidence for the enzymatic transfer of an arabinosyl residue onto linear mannoside acceptors in M. smegmatis cell-free extracts.
(a) Structures of the synthetic mannosides acceptors bearing an octyl chain (1–4) that were used to probe the novel (1→6) AraT activity in M. smegmatis extracts; structures of pRpp and the D-arabinofuranose donor, DPA.
(b) TLC analysis of the AraT reaction product formed using acceptor (1) - Lane 1, non-radioactive dimannoside acceptor (1) visualized upon spraying with α-naphthol and heating; Lane 2, TLC autoradiogram showing the product of the reaction run in the absence of acceptor (1) (negative control); Lane 3, complete assay showing a radiolabeled product migrating with a lower Rf than acceptor (1).
(c) Percentage enzymatic conversions of substrates into their arabinose-containing products. Acceptor (1) appears as the most efficient substrate in the AraT reaction. The absence of product formation with C6-deoxy (4)-modified disaccharide analog suggests that the C-6 hydroxyl group is essential for (1→6) AraT activity. EMB at a concentration of 100–200 μg/mL does not inhibit the transfer of an arabinosyl residue onto acceptor (1). The results shown are representative of at least two independent assays performed with different enzyme preparations.