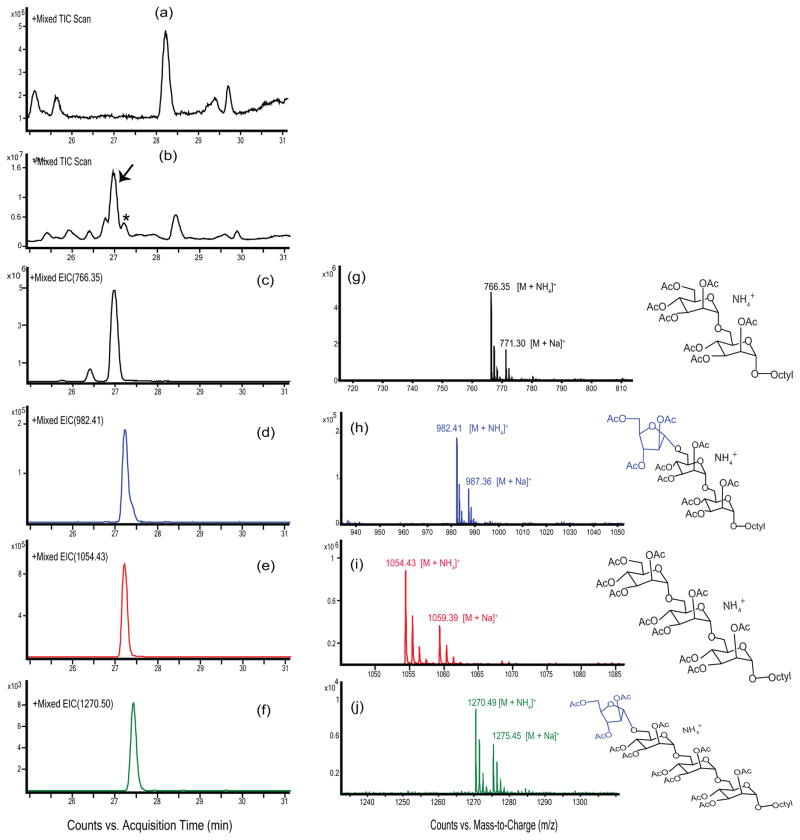
Figure 3. LC/MS analysis of the AraT reaction products.
Membrane and cell envelope preparations from M. smegmatis were incubated without arabinosyl donor or mannoside acceptor, or with DPA donor and acceptor (1). Traces (a) and (b) show the total ion chromatograms of the control lacking substrates and complete reaction, respectively. A series of peaks centered around 27 min is present in the substrate containing reaction (b) but not in the control (a). Unreacted substrate (1) was identified using selected ion monitoring of the [M+NH4]+ ion at m/z 766.34 (c); the mass spectrum is shown in (g). The mono-arabinosylated product was identified using selected ion monitoring of the [M+NH4]+ ion at m/z 982.41; the mass spectrum is shown in (h). Even though no mannose donors (GDP-Man or polyprenyl-phosphomannose) were added to the reaction, the presence of endogenous mannose donor in the membrane fraction allowed for the mono-mannosylation of acceptor (1) as shown by selected ion monitoring of the [M+NH4]+ ion at m/z 1054.43. This trimannoside was also arabinosylated as shown by selected ion monitoring of the [M+NH4]+ ion at m/z 1270.49; the mass spectrum is shown in (j). In all mass spectra, the ion at 5 amu higher mass than the [M+NH4]+ ion corresponds to the [M+Na]+ ion.