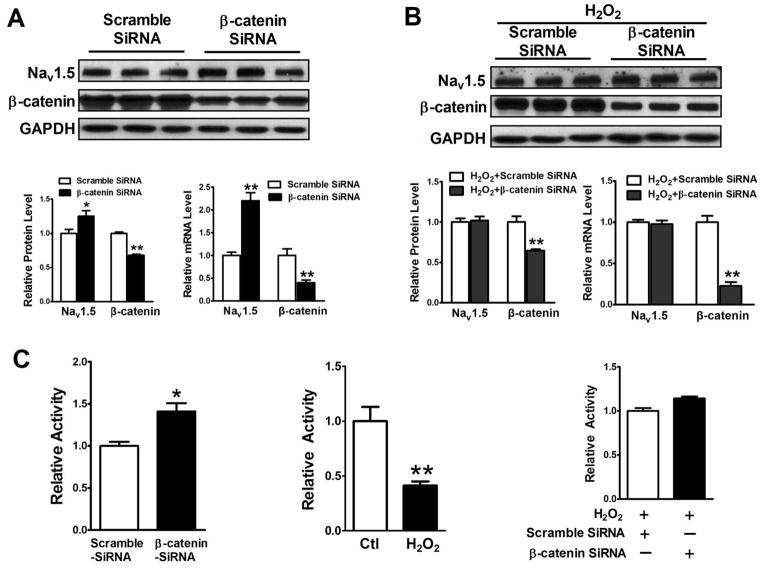
Figure 9. Knockdown of β-catenin by SiRNA prevented a decrease of both NaV1.5 expression and SCN5a promoter activity induced by H2O2.
HL-1 cells were transfected with 100 nM β-catenin and scramble SiRNA, respectively, for 48 hours without or with 48 hours 50 μM H2O2 treatment. Total Protein and RNA were extracted from these cells. Western blot and real time-PCR were performed. (A) 100 nM β-catenin SiRNA significantly knocked down β-catenin expression at both protein and mRNA levels (p<0.01) and significantly increased NaV1.5 protein and mRNA (p<0.01), compared to the 100 nM scramble SiRNA group. (B) Knockdown of β-catenin completely abolished suppressive effects of 50 μM H2O2 for 48 hours on NaV1.5 expression at both protein and mRNA levels. (C) Luciferase promoter assays were performed on the protein extracts from transfected Hela cells treated with β-catenin or scramble SiRNA or treated with or without 50 μM H2O2 for 48 hours. The results showed that β-catenin SiRNA significantly increased SCN5a promoter activity (p<0.05), compared to the scramble SiRNA control group and that H2O2 significantly inhibited SCN5a promoter activity (p<0.01), compared to the control group. In order to determine if H2O2 effects on the SCN5a promoter activity is related to β-catenin, luciferase promoter assays were performed on the protein extracts from Hela cells transfected with β-catenin or scramble SiRNA and these cells were cultured in the medium containing 50 μM H2O2. The results showed that b-catenin SiRNA completely abolished the H2O2 suppressive effects, compared to the scramble group. *p<0.05 and **p<0.01; n=3 batches of HL-1 or HeLa cells from 3 independent experiments carried on separate occasions for each group.