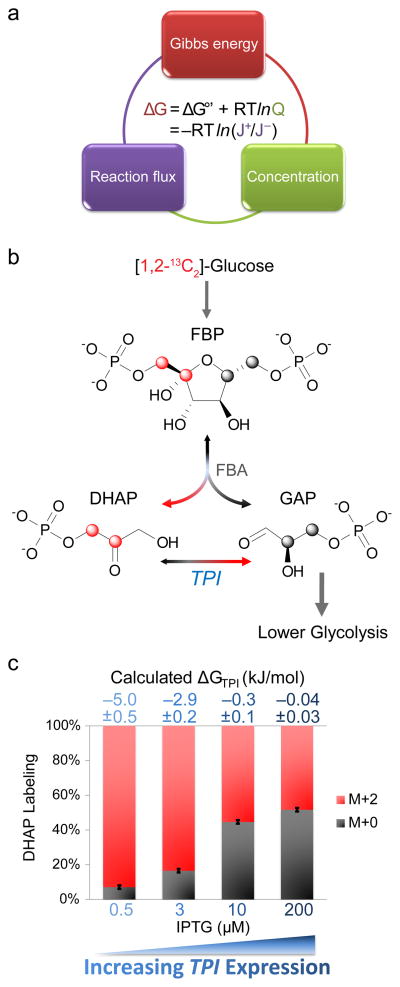
Figure 1. Tracing forward-to-backward flux through triose phosphate isomerase (TPI).
(a) The free energy of cellular reactions (ΔG) is independently determined by i) the reaction standard free energy adjusted for substrate and product concentrations and ii) the ratio of forward-to-backward fluxes. The integration of experimental measurements of forward-to-backward reaction fluxes and of metabolite concentrations results in more coherent and precise determination of both concentrations and ΔG. (b) [1,2-13C]-glucose (red atom: 13C) yields [1,2-13C]-fructose-1,6-bisphosphate (FBP), the parent molecule of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP). FBP carbons 1–3 form DHAP. Thus, in the absence of backwards flux through triose phosphate isomerase (TPI), all DHAP molecules would be labeled (M+2). Reverse flux through TPI results in the appearance of unlabeled DHAP. (c) To verify that we can measure different extents of reversibility, we knocked out in E. coli the chromosomal TPI and introduced a plasmid containing TPI under the control of inducer IPTG. The fraction of unlabeled DHAP progressively increased with IPTG addition. The extent of DHAP labeling at pseudo-steady state was used to determine the ratio of forward-to-backward TPI flux by isotopomer balancing. The flux ratio then yields ΔG as per (a). Labeling fraction error bars represent standard deviations (n=3) and calculated ΔG errors represent 95% confidence intervals.