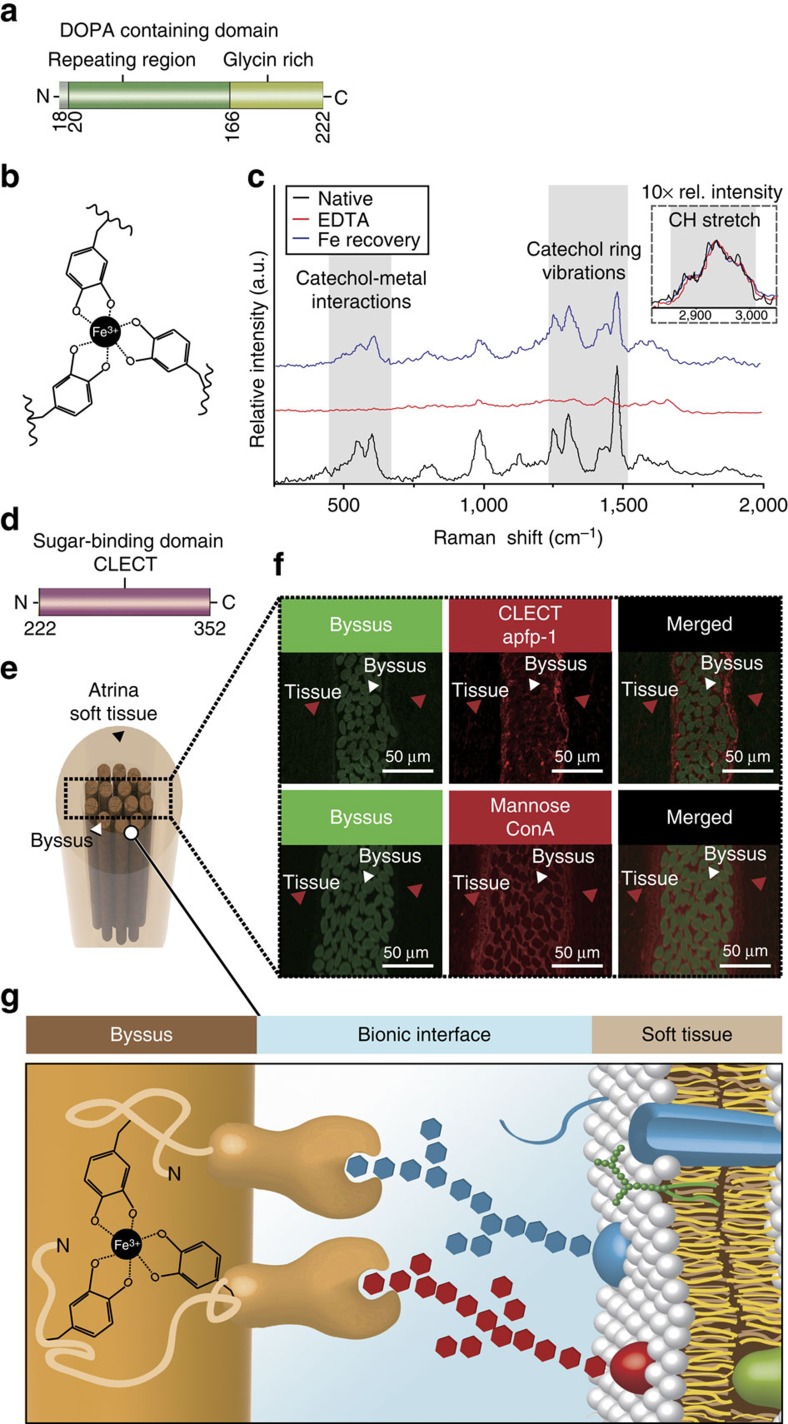
Figure 2. Role of apfp-1 in Atrina pectinata.
(a) DOPA-containing domain in apfp-1. (b) Tris-DOPA–Fe3+ complex. (c) Resonance Raman spectra from apfp-1 (black), after EDTA treatment (red) and after re-exposure to Fe in apfp-1 (blue). The nearly complete loss of resonance peaks after EDTA treatment is reversed to a nearly complete restoration by means of incubation. in 1 mM FeCl3 (pH 3.2). The three spectra were normalized to the area under the aliphatic CH peak (2,850 to 3,010 cm−1). The inset represents the relative intensity of the non-resonance peak for aliphatic CH stretching from apfp-1 magnified × 10. According to the assignments, the most prominent peaks can be attributed to the interaction of metal with the catecholic oxygens and to the vibrations of the carbon bonds in the catechol ring, respectively. (d) Sugar-binding domain in apfp-1. (e) Cross-section through the tissue surrounding the Atrina byssus. (f) Confocal imaging of Atrina byssus and the surrounding tissue labelled with anti-apfp-1 antibody to detect apfp-1, and with ConA to detect mannose. Green box represents the auto-fluorescence of the byssus which is the location that shows where the byssus fibre is. Red box represents the apfp-1 antibody to detect apfp-1, and with ConA to detect mannose. (g) The byssus is coated with apfp-1. Its DOPA–Fe3+ binding property gives stiff and extensible properties to the cuticle region, like fp-1 in mussel byssus. The apfp-1 lectin domain strongly binds to mannose in the cell membrane that surrounds the non-living byssus and acts as the bionic interface between non-living tissue and the cell.