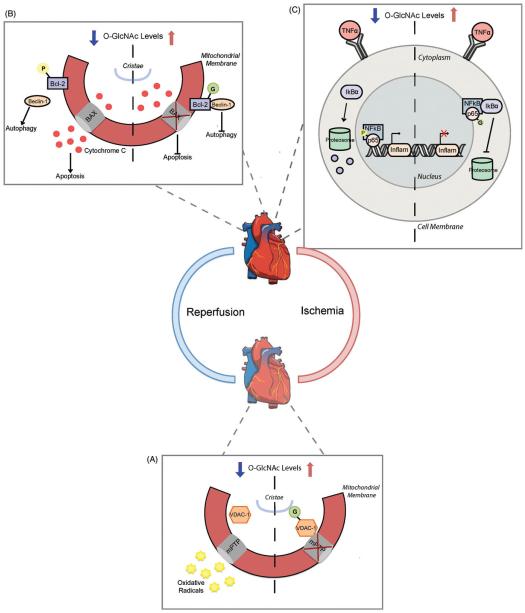
Figure 5.
Increased O-GlcNAcylation offers cardioprotection following ischemia-reperfusion injury. (A) Elevations in O-GlcNAc after vascular ischemia limit oxidative stress through a mitochondrial VDAC-1 mechanism. O-GlcNAc modification of VDAC-1 increases its interaction with the mitochondria permeability transition pore (mPTP) and prevents radical release. When VDAC-1 is unmodified, the mPTP can open and release harmful radical species into circulation. B Upon cardiac reperfusion the pro-autophagic protein Beclin-1 dissociates from its inhibitor Bcl-2 and stimulates constitutively active autophagy. Phosphorylation of Bcl-2 prevents its interaction with Bcl-2 associated X protein (BAX) in the mitochondrial membrane, causing cytochrome c release and apoptosis signal initiation. Bcl-2 O-GlcNAcylation during reperfusion promotes its interaction with Beclin-1 and BAX to inhibit downstream activation of autophagy and apoptosis pathways. (C) NFkB signaling is common following reperfusion in the heart. Decreasing O-GlcNAc promotes phosphorylation of the NFkB DNA binding subunit p65 and restricts IkBa protein inhibition. This enables p65 nuclear translocation where it can stimulate inflammatory gene activation. O-GlcNAc modified p65 subsequently blocks its phosphorylation to promote IkBa-mediated NFkB inhibition and prevents inflammatory gene activation. (see colour version of this figure at www.informahealthcare.com/bmg).