Abstract
Background & Aims
The high costs of direct-acting antiviral (DAA) agents to treat chronic hepatitis C virus (HCV) infection have resulted in denials of treatment, but it is not clear whether patients’ access to these therapies differs with their type of insurance.
Methods
We conducted a prospective cohort study among all patients who had a DAA prescription submitted between November 1, 2014 and April 30, 2015 to Burman’s Specialty Pharmacy, which provides HCV pharmacy services to patients in Delaware, Maryland, New Jersey, and Pennsylvania. We determined the incidence of absolute denial of DAA prescription, defined as lack of approval of prescription fill by the insurer, according to type of insurance (US Medicaid, US Medicare, commercial insurance). Multivariable Poisson regression was used to estimate adjusted relative risks (RRs) of absolute denial associated with patient characteristics.
Results
Among 2321 patients prescribed a DAA regimen (503 covered by Medicaid; 795 by Medicare; 1023 by commercial insurance), 377 (16.2%) received an absolute denial. The most common reasons for absolute denial were insufficient information to assess medical need (134 [35.5%]) and lack of medical necessity (132 [35.0%]). A higher proportion of patients covered by Medicaid received an absolute denial (233 [46.3%]) than those covered by Medicare (40 [5.0%]; P<.001) or commercial insurance (104 [10.2%]; P<.001). Medicaid insurance (adjusted RR, 4.14; 95% confidence interval, 3.38–5.08) and absence of cirrhosis (adjusted RR, 1.96; 95% confidence interval, 1.53–2.50) were associated with absolute denial.
Conclusions
There are significant disparities in access to DAA-based treatments for HCV infection among patients with different types of insurance. Nearly half of Medicaid beneficiaries in Delaware, Maryland, New Jersey, and Pennsylvania were denied access to these drugs for chronic HCV infection.
Keywords: hepatitis C, direct-acting antiviral, insurance
Over 3.2 million people in the US are chronically infected with hepatitis C virus (HCV) infection.1 If left untreated, chronic HCV can cause progressive liver fibrosis and cirrhosis, leading to hepatic decompensation and hepatocellular carcinoma.2 Viral eradication after antiviral therapy reduces the risk of liver complications and death, even with advanced hepatic fibrosis.3 Consequently, HCV treatment guidelines have recommended antiviral therapy for all chronic HCV-infected patients.4,5
Highly efficacious direct-acting antiviral (DAA) agents were approved by the US Food and Drug Administration in 2014 to treat chronic HCV.6–8 However, their high costs have led insurers to restrict access to these medications,9–12 requiring that patients meet insurer-specific criteria for approval, such as evidence of advanced liver fibrosis, consultation with a specialist, or abstinence from alcohol or illicit drug use.13,14 Two recent reports highlighted the restrictions on reimbursement of DAAs across the US state Medicaid programs and revealed considerable heterogeneity by state in the criteria for approval.15,16 Little is known about restrictions to HCV treatment among US Medicare and commercial insurance beneficiaries.
As a consequence of these varying restrictions, insurers have required that DAA prescriptions undergo prior authorization, a pre-approval process to determine if the patient meets insurer-specific criteria for HCV treatment. Prescriptions may be denied after this review, but the decision can be appealed by the provider. The insurer may overturn the denial, if given sufficient supporting information, or uphold the decision. DAA prescriptions that ultimately are not filled because of a lack of insurer approval are considered absolutely denied. Data are lacking on the incidence of absolute denial of DAA prescription and factors associated with this outcome in clinical practice settings. These data are important because absolute denial of HCV treatment by insurers might have adverse outcomes on patients and could harm patient-provider relationships.
We evaluated the incidence of absolute denial of DAA therapy among a sample of US chronic HCV-infected patients by type of insurance. Since the criteria for reimbursement of DAA medications may be more restrictive within the Medicaid program than within other types of insurance,15,16 we hypothesized that absolute denial of DAA treatment would be more common among Medicaid beneficiaries. We also evaluated the reasons for absolute denial given by the insurers, factors associated with absolute denial, and time to fill among those whose prescription was approved.
METHODS
Study Design and Data Source
We conducted a prospective cohort study using data from Burman’s Specialty Pharmacy, which provides HCV pharmacy services to community and academic medical practices across Delaware, Maryland, New Jersey, and Pennsylvania. DAAs are often dispensed by specialty pharmacies because of their high costs and requirements for special handling and delivery.17 Burman’s obtains medical information from clinicians to complete the prior authorization request and submits the prescription and request to the insurer. Burman’s uses an electronic record system to collect data on demographics, health insurance, and prescribed medications. Clinical information submitted to the pharmacy by the clinician for the prior authorization request, including documentation of hepatic fibrosis stage, human immunodeficiency virus (HIV) coinfection, and previous HCV treatment and response, is also electronically recorded. Burman’s collects information from prescribing clinicians on alcohol or drug use when requested for the prior authorization. The study was approved by the University of Pennsylvania Institutional Review Board.
Study Patients
Patients were included if they were infected with HCV genotype 1, 2, or 3 (the most common HCV genotypes in the US18) and had a DAA prescription submitted to the pharmacy between November 1, 2014 and April 30, 2015 (the first six months that interferon-containing regimens were no longer recommended as first-line therapy4). Patients were excluded if their prior authorization was completed by an outside pharmacy (since medical information might not be available to Burman’s), their insurer mandated use of a different pharmacy, or they had no health insurance. If a patient had multiple DAA treatment courses prescribed during the period of interest, only the first regimen was included.
Main Study Outcomes
The primary outcome was absolute denial of DAA prescription, defined as lack of approval of DAA fill by the insurer, even after appeal. Burman’s ascertained the status of all prescriptions with the insurers through September 30, 2015.
As secondary outcomes, we evaluated: 1) the reason given by the insurer for absolute denial, 2) denial preceding prescription approval, 3) any denial (composite of either absolute denial or denial preceding insurer approval), 4) time to DAA fill (days from receipt of the DAA prescription by the pharmacy to the date of fill), and 5) time to absolute denial (days from receipt of the DAA prescription by the pharmacy to the date of absolute denial).
Data Collection
Demographic and clinical data collected from Burman’s electronic records at the time the DAA prescription was received by the pharmacy included: age; sex; race; insurance; HCV RNA; HCV genotype; presence of cirrhosis (based on clinician report from liver biopsy or non-invasive test); history of HCV treatment and response (based on prior prescription fills for antiviral therapy and/or clinician report); HIV status (reported by clinician); DAA regimen prescribed; and date of DAA prescription receipt by the pharmacy. Insurance was classified as US Medicaid (joint federal- and state-funded programs for medical care and drug benefits for low-income and special-needs individuals19), US Medicare (federal health insurance program available to Americans aged ≥65 years and to those under 65 years with certain disabilities or chronic health conditions20), or commercial insurance (health benefits that are employer-sponsored, privately purchased, or obtained via health exchange through the Affordable Care Act21). Patients were classified according to the insurance plan to which the DAA prescription was submitted. Patients covered by Medicaid fee-for-service or Medicaid managed care were classified as having Medicaid insurance.
Data collected after receipt of the DAA prescription included dates of: completion of prior authorization, insurer denial preceding approval, absolute denial by insurer, appeal of the insurer’s decision by the clinician, and DAA fill.
Statistical Analysis
Follow-up began on the date that the DAA prescription was received by the specialty pharmacy and continued until the pharmacy ascertained the final outcome for the prescription (i.e., absolute denial, DAA prescription fill) or determined that the prior authorization request was incomplete (i.e., after 60 days of inactivity). Patients who had an incomplete prior authorization were excluded from analyses since a completed prior authorization is required for insurer review and a decision to either approve or deny the DAA prescription.
The incidence of absolute denial of DAA prescription was determined, overall and by type of insurance, cirrhosis status, and HCV genotype. The reason given by the insurer for the absolute denial was evaluated. We also calculated the incidence of denial preceding prescription approval and of any DAA denial, by insurance type.
Next, we used multivariable Poisson regression with a robust error variance to estimate the relative risks (RRs) with 95% confidence intervals (CIs) of absolute denial associated with patient factors.22 We used Poisson, rather than logistic, regression because odds ratios may overestimate RRs when the outcome of interest is common, as in this study.23 We hypothesized that Medicaid coverage and absence of cirrhosis would be the strongest determinants of absolute denial. Other variables evaluated within the multivariable model included age, sex, race, genotype, prior HCV treatment, HIV, and time period of DAA prescription (DAA prescribed within the first three months of the observation period versus latter three months). To avoid bias from missing data, we implemented multiple imputation using chained equations.24 Twenty imputed datasets were created using all of the variables from the Poisson model, including absolute denial status. Results across the 20 datasets were combined to arrive at CIs that accounted for within- and across-dataset variances.25
Finally, we determined the median time to DAA fill, by insurance. Results were stratified according to receipt of insurer denial prior to approval. The median time to absolute denial was also calculated. Data were analyzed using Stata 12.1 (Stata Corporation, College Station, TX).
RESULTS
Study Patients
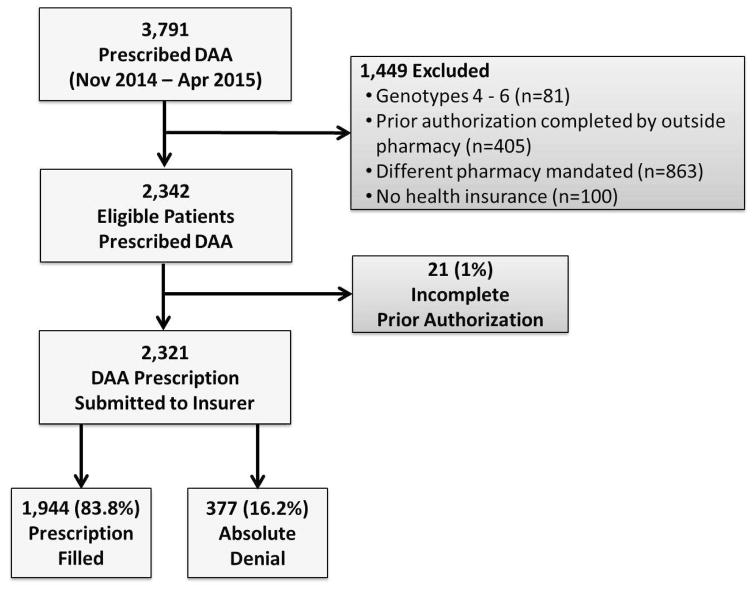
Between November 1, 2014 and April 30, 2015, Burman’s received DAA prescriptions for 3,791 patients. After exclusions (Figure 1), 2,342 patients remained. Among these, 21 (0.9%) had an incomplete prior authorization after 60 days and were excluded, leaving 2,321 patients (503 with Medicaid [492 (97.8%) Medicaid managed care; 11 (2.2%) Medicaid fee-for-service]; 795 with Medicare; 1,023 with commercial insurance). Medicaid patients were younger, more commonly black, and more frequently HCV treatment-naïve than those with Medicare or commercial insurance (Table 1). Cirrhosis and HIV coinfection were more frequent among patients with Medicaid and Medicare than commercial insurance (p<0.01 for all comparisons). HCV genotype 1 was the most common genotype across the insurance types.
Figure 1.
Selection flow of patients prescribed a direct-acting antiviral (DAA) into the study cohort.
Table 1.
Characteristics of chronic hepatitis C virus (HCV)-infected patients for whom a direct-acting antiviral prescription was received by the pharmacy, overall and by type of insurance.
| Characteristic | Overall (n=2,321) | US Medicaid (n=503) | US Medicare (n=795) | Commercial Insurance (n=1,023) | P-value | |
|---|---|---|---|---|---|---|
| Medicaid versus Medicare | Medicaid versus Commercial | |||||
| Median age (years, IQR) | 58 (52 – 63) | 55 (49 – 60) | 60 (54 – 66) | 58 (52 – 62) | <0.001 | <0.001 |
| Female sex (n, %) | 918 (39.6) | 218 (43.3) | 323 (40.6) | 377 (36.8) | 0.33 | 0.02 |
| Race (n, %) | ||||||
| Black or African American | 608 (26.2) | 166 (33.0) | 233 (29.3) | 209 (20.4) | <0.001 | <0.001 |
| White | 1,416 (61.0) | 208 (41.4) | 480 (60.4) | 728 (71.2) | ||
| Asian | 17 (0.7) | 8 (1.6) | 4 (0.5) | 5 (0.5) | ||
| Other/Unknown | 280 (12.1) | 121 (24.1) | 78 (9.8) | 81(7.9) | ||
| HCV genotype (n, %) | ||||||
| 1 | 2,114 (91.1) | 445 (88.5) | 733 (92.2) | 936 (91.5) | 0.02 | 0.06 |
| 2 or 3 | 207 (8.9) | 58 (11.5) | 62 (7.8) | 87 (8.5) | ||
| Median HCV RNA (log IU/mL, IQR) | 6.3 (5.8 – 6.7) | 6.2 (5.8 – 6.6) | 6.3 (5.8 – 6.7) | 6.3 (5.8 – 6.7) | 0.11 | 0.24 |
| Cirrhosis (n %) | 715 (30.8) | 165 (32.8) | 281 (35.4) | 269 (26.3) | 0.30 | 0.01 |
| Unknown | 370 (16.2) | 77 (15.3) | 124 (15.6) | 176 (17.2) | ||
| Prior HCV treatment response (n, %) | ||||||
| No prior treatment | 1,572 (67.7) | 391 (77.7) | 510 (64.2) | 671 (65.6) | <0.001 | <0.001 |
| Non-response | 366 (15.8) | 40 (8.0) | 153 (19.2) | 173 (16.9) | ||
| Partial response | 89 (3.8) | 11 (2.2) | 34 (4.3) | 44 (4.3) | ||
| Relapse | 180 (7.8) | 26 (5.2) | 65 (8.2) | 89 (8.7) | ||
| Unknown | 114 (4.9) | 35 (7.0) | 33 (4.2) | 46 (4.5) | ||
| HIV coinfection (n, %) | 92 (4.0) | 29 (5.8) | 41 (5.2) | 22 (2.2) | 0.66 | <0.001 |
| Regimen prescribed (n, %) | ||||||
| Sofosbuvir-ledipasvir +/− ribavirin | 1,953 (84.1) | 399 (79.3) | 680 (85.5) | 874 (85.4) | 0.03 | 0.002 |
| Sofosbuvir + ribavirin | 210 (9.0) | 57 (11.3) | 64 (8.0) | 89 (8.7) | ||
| Sofosbuvir+ simeprevir +/− ribavirin | 66 (2.8) | 22 (4.4) | 27 (3.4) | 17 (1.7) | ||
| Paritaprevir/ritonavir-ombitasvir + dasabuvir +/− ribavirin | 62 (2.7) | 16 (3.2) | 11 (1.4) | 35 (3.4) | ||
| Pegylated interferon alfa + sofosbuvir + ribavirin | 30 (1.3) | 9 (1.8) | 13 (1.6) | 8 (0.8) | ||
Abbreviations: HCV=hepatitis C virus; HIV=human immunodeficiency virus; IQR=interquartile range; RNA=ribonucleic acid
Incidence of Absolute Denial
Among these 2,321 patients, 377 (16.2%; 95% CI, 14.8–17.8%) were absolutely denied their DAA prescription. Absolute denial was more common for patients with Medicaid (233 [46.3%]) than Medicare (40 [5.0%]; p<0.001) or commercial insurance (104 [10.2%]; p<0.001; Figure 2). When the analysis was restricted to the 715 patients with cirrhosis, the incidence of absolute denial remained higher for patients with Medicaid (42/165 [25.4%]) than Medicare (4/281 [1.4%]; p<0.001) or commercial insurance (8/269 [3.0%]; p<0.001). Among Medicaid beneficiaries, no statistically significant difference in absolute denial rate was observed by state (Delaware: 8/14 [57.1%]; Maryland: 8/17 [47.1%]; New Jersey: 35/94 [37.2%]; Pennsylvania: 182/378 [48.2%]; p=0.06). Absolute denial of DAA prescription was more frequent among patients with HCV genotype 3 (24/76 [31.6%]) than genotype 1 (327/2,114 [15.5%]; p<0.001) but not significantly different compared to genotype 2 (26/131 [19.9%]; p=0.06). There was no difference in the incidence of absolute denial between patients with genotype 1 and 2 (p=0.18).
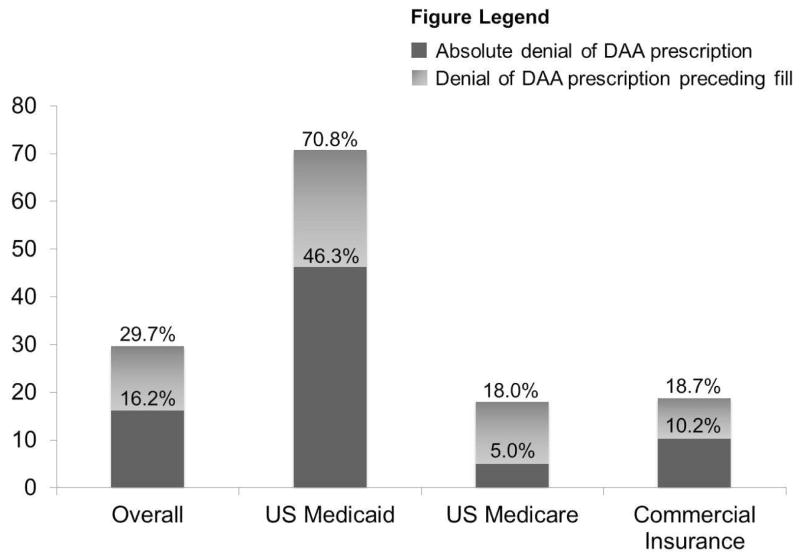
Figure 2.
Incidence of any denial of a direct-acting antiviral (DAA) prescription by the insurer (overall height of bar), insurer denial of a DAA prescription preceding approval (light gray bar), and absolute denial of a DAA prescription by the insurer (dark gray bar), overall and by insurance status.
Table 2 reports the incidence of DAA denial preceding insurer approval. When the composite of either absolute denial or denial preceding approval (i.e., any denial) was evaluated, 690 patients (29.7%; 95% CI, 27.9–31.6%) received a denial of DAA treatment. Receipt of any denial was nearly 4-fold more common for Medicaid (356 [70.8%]) than Medicare (143 [18.0%]; p<0.001) or commercially insured patients (191 [18.7%]; p<0.001; Figure 2). Among patients issued any denial, an appeal of the insurer’s decision by the clinician was less commonly filed for patients with Medicaid (38/356 [10.7%]) than Medicare (39/143 [27.3%]; p<0.001) or commercial insurance (41/191 [21.5%]; p<0.001).
Table 2.
Outcomes of prescription of direct-acting antiviral therapy.
| P-value | ||||||
|---|---|---|---|---|---|---|
| Event | Overall (n=2,321) | US Medicaid (n=503) | US Medicare (n=795) | Commercial (n=1,023) | Medicaid vs. Medicare | Medicaid vs. Commercial |
|
| ||||||
| No denial of DAA prescription by insurer | 1,631 (70.3%) | 147 (29.2%) | 652 (82.0%) | 832 (81.3%) | <0.001 | <0.001 |
| Any denial of DAA by insurer | 690 (29.7%) | 356 (70.8%) | 143 (18.0%) | 191 (18.7%) | <0.001 | <0.001 |
| Insurer denial of DAA preceding fill | 313 (13.5%) | 123 (24.5%) | 103 (13.0%) | 87 (8.5%) | <0.001 | <0.001 |
| Absolute denial of DAA by insurer | 377 (16.2%) | 233 (46.3%) | 40 (5.0%) | 104 (10.2%) | <0.001 | <0.001 |
| Reason reported by insurer | <0.001 | <0.001 | ||||
| Insufficient information to assess medical need | 134 (35.5%) | 112 (48.1%) | 7 (17.5%) | 16 (15.4%) | ||
| Lack of medical necessity | 132 (35.0%) | 72 (30.9%) | 12 (30.0%) | 48 (46.2%) | ||
| Positive drug screen | 15 (4.0%) | 9 (3.9%) | 0 (0%) | 6 (5.8%) | ||
| Not preferred DAA | 10 (2.7%) | 2 (0.8%) | 4 (10.0%) | 4 (3.8%) | ||
| Unknown, no denial letter received by pharmacy | 86 (22.8%) | 38 (16.3%) | 17 (42.5%) | 30 (28.8%) | ||
| DAA prescription ultimately filled | 1,944 (83.8%) | 270 (53.7%) | 755 (95.0%) | 919 (89.8%) | <0.001 | <0.001 |
Abbreviation: DAA, direct-acting antiviral
Table 2 reports the frequency of DAA prescription approval and absolute denial categorized by reason reported by the insurer, according to type of insurance. Overall, the most common reasons for absolute denial were insufficient information to assess medical need (134 [35.5%]) and lack of medical necessity (132 [35.0%]), and these were the most frequently reported reasons among Medicaid beneficiaries as well.
Factors Associated with Absolute Denial
In the multivariable analysis, Medicaid insurance, absence of cirrhosis, and DAA prescription in the initial three months of the 6-month observation period were associated with a higher risk of absolute denial (Table 3). Higher age and Medicare coverage were associated with a lower risk of absolute denial. Sex, race, HCV genotype, prior HCV treatment, and HIV coinfection were not associated with absolute denial. HCV genotype 3 was not associated with an increased risk of absolute denial in multivariable analysis.
Table 3.
Factors associated with absolute denial of a prescription for direct-acting antiviral (DAA)-based hepatitis C virus (HCV) treatment regimens (n=2,321).
| Factor | Unadjusted Relative Risk of Absolute Denial (95% CI) | Adjusted Relative Risk of Absolute Denial (95% CI) |
|---|---|---|
| Age (per year increase) | 0.96 (0.95 – 0.96) | 0.98 (0.97 – 0.98) |
| Type of insurance | ||
| Commercial | Ref | Ref |
| US Medicare | 0.49 (0.35 – 0.70) | 0.61 (0.43 – 0.86) |
| US Medicaid | 4.56 (3.71 – 5.59) | 4.14 (3.38 – 5.08) |
| Cirrhosis | ||
| Present | Ref | Ref |
| Absent | 2.54 (1.94 – 3.34) | 1.96 (1.53 – 2.50) |
| HIV coinfection | ||
| Absent | Ref | Ref |
| Present | 0.64 (0.37 – 1.13) | 0.69 (0.41 – 1.16) |
| Sex | ||
| Male | Ref | Ref |
| Female | 1.27 (1.05 – 1.53) | 1.04 (0.89 – 1.22) |
| Race | ||
| Non-black | Ref | Ref |
| Black | 0.87 (0.68 – 1.11) | 0.87 (0.68 – 1.11) |
| Genotype | ||
| 1 | Ref | Ref |
| 2 | 1.29 (0.90 –1.84) | 0.95 (0.71 – 1.27) |
| 3 | 2.04 (1.45 – 2.88) | 1.05 (0.82 – 1.36) |
| Prior HCV therapy | ||
| Previously treated | Ref | Ref |
| Treatment-naïve | 1.75 (1.38 – 2.20) | 1.16 (0.94 – 1.43) |
| Calendar period | ||
| 2/1/2015 – 4/30/2015 | Ref | Ref |
| 11/1/2014 – 1/31/2015 | 3.57 (2.65 – 4.79) | 2.33 (1.77 – 3.07) |
Abbreviations: CI=confidence interval; DAA=direct-acting antiviral; HCV=hepatitis C virus; HIV=human immunodeficiency virus; Ref, reference
Median Time to Prescription Fill
The median time to DAA fill was longer for Medicaid than Medicare or commercially insured patients (Table 4). Among patients who had a denial preceding insurer approval, the median time to fill was substantially longer for all insurance types, but remained greater for Medicaid patients. The median time to absolute denial was shorter for patients with Medicaid and Medicare than commercial insurance.
Table 4.
Median time to prior authorization request completion and direct-acting antiviral prescription fill and absolute denial, overall and by type of insurance.
| Median Days to Fill (IQR) | P-value | |||||
|---|---|---|---|---|---|---|
| Overall (n=2,321) | Medicaid (n=503) | Medicare (n=795) | Commercial (n=1,023) | Medicaid vs. Medicare | Medicaid vs. Commercial | |
| Median time to prior authorization completion (days, IQR) | 9 (6 – 13) | 9 (7 – 14) | 9 (7 – 13) | 9 (6 – 13) | 0.68 | 0.16 |
|
| ||||||
| Median time to prescription fill (days, IQR) | ||||||
| All approved prescriptions | 14 (10 – 24) | 24 (13 – 49) | 14 (10 – 21) | 14 (9 – 22) | <0.001 | <0.001 |
| Insurer denial preceding approval of prescription fill | 38 (25 – 60) | 46 (32 – 68) | 35 (21 – 51) | 32 (25 – 59) | <0.001 | 0.003 |
| Prescription fill approved without insurer denial | 13 (9 – 18) | 14 (8 – 21) | 13 (9 – 17) | 13 (9 – 19) | 0.33 | 0.70 |
|
| ||||||
| Median time to absolute denial (days, IQR) | 15 (10 – 22) | 14 (9 – 21) | 11 (8 – 20) | 18 (12 – 28) | 0.263 | 0.006 |
Abbreviation: IQR=interquartile range
DISCUSSION
In this study of chronic HCV-infected patients prescribed DAA-based HCV therapy across Delaware, Maryland, New Jersey, and Pennsylvania between November 2014 and April 2015, 16% were absolutely denied treatment by their insurance carrier. Notably, 46% of Medicaid beneficiaries from these states did not have their prescription approved for fill, and this was substantially higher than those with Medicare or commercial insurance. The disparity was even more evident among those with cirrhosis, with 25% of Medicaid beneficiaries absolutely denied treatment compared to almost none of those with other types of insurance. Lack of medical necessity and incomplete data to determine medical need were the most frequently reported reasons for absolute denial among Medicaid beneficiaries. Finally, Medicaid patients experienced a longer time to prescription fill than those with Medicare or commercial insurance. These data confirm the effects of restrictive pre-approval policies for new HCV treatments and provide concerning evidence of a disparity in access to HCV treatment.
The high incidence of DAA prescription denials among Medicaid beneficiaries in this study, along with the longer time to fill, is likely a direct consequence of the restrictive criteria for approval of these drugs that have been implemented across state Medicaid programs, which has been highlighted in recent reports.15,16 Faced with the high cost of DAAs, limited budgets, and the potential that future regimens currently being studied in clinical trials may decrease drug prices through competition, state-run Medicaid programs have elected to prioritize certain groups over others when deciding whether to allocate DAA treatments. One review found that 74% of Medicaid programs required evidence of advanced hepatic fibrosis or cirrhosis, 69% requested prescription by or consultation with a specialist, and 50% required a period of abstinence from drugs and/or alcohol.15 Our study’s findings that Medicaid coverage and lack of cirrhosis were important factors associated with absolute denial are consistent with these reports.
Lack of medical necessity was a frequent reason reported by insurers for absolute denial of DAA therapy. During the 6-month observation period covered by this study, guidelines issued by the American Association for the Study of Liver Diseases and Infectious Diseases Society of America recommended antiviral treatment for all patients with chronic HCV, but prioritized DAA-based HCV therapy for certain subgroups, particularly those with advanced hepatic fibrosis or cirrhosis.4 As of October 2015, these HCV treatment guidelines no longer provide prioritizations for DAA therapy.5 DAA treatments have also been shown to be cost-effective in recent analyses.26–28 Our finding that prescription of DAA treatment in the latter three months of our observation period was less likely to be associated with denial may suggest that insurers are relaxing criteria for reimbursement over time.
Medicaid patients were also commonly denied treatment due to insufficient information to assess medical need. It is unclear why so many patients were denied for this reason since these patients had complete prior authorization requests that should have contained the materials needed to justify approval. In most instances, the specific information that was missing was not reported in the denial letter to the clinician, making it difficult to appeal the decision. This lack of specificity might have been the reason that fewer appeals were filed by providers caring for Medicaid patients. Future studies should also investigate whether providers for Medicaid patients are less able to navigate through the prior authorization process and if information required from Medicaid patients is different from those with other types of insurance.
The implications of absolute denial of DAA treatment remain unknown. However, patients denied access to new DAA therapies may have continued progression of hepatic fibrosis and remain at risk for the development of cirrhosis, end-stage liver disease, and hepatocellular carcinoma. Indeed, a recent analysis using data from the Veterans Health Administration suggests that deferring anti-HCV therapy until the development of advanced hepatic fibrosis/cirrhosis reduces treatment effectiveness and increases risk of liver-related complications and death.29 A separate analysis among HIV/HCV-coinfected patients in the Swiss HIV Cohort Study found that deferring treatment from METAVIR stage F2 until stage F3 or F4 increased the risk of liver-related death 2-fold and 5-fold, respectively.30 Denial of DAA treatment can also lead to ongoing HCV-associated inflammation, which might increase the risk of extra-hepatic complications, including bone,31,32 kidney,33,34 cardiovascular,35 and neuropsychiatric disease.36 Further, failure to treat and cure chronic HCV can lead to continued risk of HCV transmission. Denial of DAA therapy might also promote anxiety and stress about HCV disease progression and provoke distrust among patients of the healthcare system and their providers. Clinicians are then challenged to explain the denial, and important opportunities for patient engagement, education, and cure could be irrevocably lost.
This study had several potential limitations. Since Medicaid programs have different criteria for DAA prescription, our findings among the Medicaid patients within Delaware, Maryland, New Jersey, and Pennsylvania might not be generalizable to beneficiaries in other states.37 Our results might also not be generalizable to patients covered by integrated health plans. Further, our analysis included a sample of chronic HCV-infected patients from one specialty pharmacy in the US Mid-Atlantic region and may not be representative of chronic HCV patients nationally or reflect prescription outcomes at other pharmacies dispensing DAA therapy. However, the characteristics of the patients in this study are similar to those within recent DAA treatment trials6–8 and observational studies of chronic HCV-infected patients.1,38
Moreover, this study evaluated access to DAA therapy during the period when the first all-oral DAA regimens were available and the market was dominated by one supplier. The recent release of several new DAAs (e.g., daclatasvir, elbasvir/grazoprevir) appears to be resulting in greater price competition, which could allow greater access to these agents in the future. In addition, on November 5, 2015, the Centers for Medicare and Medicaid Services notified states that restricting access to DAA drugs is contrary to the statutory requirements within section 1927 of the Social Security Act.39 They also sent letters to DAA drug manufacturers inquiring about opportunities for discount or value-based purchasing arrangements to make these medications more affordable. The long-term effects of an expanding supply of agents and pressure from government sources on treatment access remain to be seen.
In conclusion, most Medicare and commercial insurance beneficiaries have access to DAA-based treatment for chronic HCV infection, but nearly half of the Medicaid beneficiaries within Delaware, Maryland, New Jersey, and Pennsylvania were denied access. Notably, nearly one quarter of Medicaid recipients with cirrhosis experienced treatment denial. Medicaid patients from these states also experienced a longer time to prescription fill than those with Medicare or commercial insurance. These data show that the restrictive pre-approval policies for DAA therapies among Medicaid beneficiaries have led to an important disparity in access to HCV therapy that must be addressed.
Acknowledgments
Grant Support: This research was supported by grant funding from the Penn Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 045008).
Abbreviations
- CI
confidence interval
- DAA
direct-acting antiviral
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- RR
relative risk
Footnotes
Financial Disclosures: Dr. Lo Re has received investigator-initiated research grant support (to the University of Pennsylvania) from AstraZeneca. Ms. Gilmore has served on the advisory boards of AbbVie, Bristol-Myers Squibb, and Gilead Sciences. Dr. Doshi has served on the advisory boards of Alkermes, Boehringer Ingelheim, Forest, Ironwood Pharmaceuticals, Merck, and Shire; has received research grant support (to the University of Pennsylvania) from Amgen, Merck, Pfizer, PhRMA, and the National Pharmaceutical Council; and has a spouse who holds stocks in Merck and Pfizer. Dr. Reese has received investigator-initiated research grant support from Merck. Dr. Reddy has served on the advisory boards of Merck, AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Janssen and has received research grant support (to the University of Pennsylvania) from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, and Merck. Dr. Kostman has served on the advisory board of Gilead Sciences. Dr. Urick, Mr. Halladay, Ms. Battista, and Ms. Peleckis are employees of Burman’s Specialty Pharmacy. All other authors report no conflicts of interest.
Author Contributions:
Study concept and design: Lo Re, Kostman
Acquisition of data: Lo Re, Urick, Halladay, Binkley, Battista, Peleckis
Statistical analysis: Lo Re, Gowda, Roy
Analysis and interpretation of data: Lo Re, Gowda, Urick, Roy, Kostman
Drafting of the manuscript: Lo Re, Gowda, Urick, Kostman
Critical revisions of the manuscript: Lo Re, Gowda, Urick, Halladay, Binkley, Carbonari, Battista, Peleckis, Gilmore, Roy, Doshi, Reese, Reddy, Kostman
Administrative, technical, or material support: Lo Re, Carbonari
Study supervision: Lo Re
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 4.American Association for the Study of Liver Diseases/Infectious Diseases Society of America/International Antiviral Society-USA. [Accessed on November 9, 2015];Recommendations for testing, managing, and treating hepatitis C. Accessed at: http://www.hcvguidelines.org/
- 5.American Association for the Study of Liver Diseases/Infectious Diseases Society of America/International Antiviral Society-USA. [Accessed on November 14, 2015];Hepatitis C guidance underscores the importance of treating HCV infection: Panel recommends direct-acting drugs for nearly all patients with chronic hepatitis C. Accessed at: http://hcvguidelines.org/sites/default/files/when-and-in-whom-to-treat-press-release-october-2015.pdf.
- 6.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 7.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 8.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 9.Steinbrook R, Redberg RF. The high price of the new hepatitis C virus drugs. JAMA Intern Med. 2014;174:1172. doi: 10.1001/jamainternmed.2014.2135. [DOI] [PubMed] [Google Scholar]
- 10.Saag MS. Quantum leaps, microeconomics, and the treatment of patients with hepatitis C and HIV coinfection. JAMA. 2014;312:347–8. doi: 10.1001/jama.2014.7735. [DOI] [PubMed] [Google Scholar]
- 11.Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312:593–4. doi: 10.1001/jama.2014.8897. [DOI] [PubMed] [Google Scholar]
- 12.Trooskin SB, Reynolds H, Kostman JR. Access to Costly New Hepatitis C Drugs: Medicine, Money, and Advocacy. Clin Infect Dis. 2015 doi: 10.1093/cid/civ677. [DOI] [PubMed] [Google Scholar]
- 13.Simon RE, Pearson SD, Hur C, Chung RT. Tackling the hepatitis C cost problem: A test case for tomorrow's cures. Hepatology. 2015;62:1334–6. doi: 10.1002/hep.28157. [DOI] [PubMed] [Google Scholar]
- 14.Grebely J, Haire B, Taylor LE, et al. Excluding people who use drugs or alcohol from access to hepatitis C treatments - Is this fair, given the available data? J Hepatol. 2015;63:779–82. doi: 10.1016/j.jhep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann Intern Med. 2015;163:215–23. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 16.Canary LA, Klevens RM, Holmberg SD. Limited Access to New Hepatitis C Virus Treatment Under State Medicaid Programs. Ann Intern Med. 2015;163:226–8. doi: 10.7326/M15-0320. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch BR, Balu S, Schulman KA. The impact of specialty pharmaceuticals as drivers of health care costs. Health Aff (Millwood) 2014;33:1714–20. doi: 10.1377/hlthaff.2014.0558. [DOI] [PubMed] [Google Scholar]
- 18.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy S, Bilker WB, Weber A, Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol Drug Saf. 2003;12:103–11. doi: 10.1002/pds.765. [DOI] [PubMed] [Google Scholar]
- 20.Blumenthal D, Davis K, Guterman S. Medicare at 50--moving forward. N Engl J Med. 2015;372:671–7. doi: 10.1056/NEJMhpr1414856. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372:2451–8. doi: 10.1056/NEJMhpr1503614. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989–91. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newgard CD, Lewis RJ. Missing Data: How to Best Account for What Is Not Known. JAMA. 2015;314:940–1. doi: 10.1001/jama.2015.10516. [DOI] [PubMed] [Google Scholar]
- 25.Freedman VA, Wolf DA. A case study on the use of multiple imputation. Demography. 1995;32:459–70. [PubMed] [Google Scholar]
- 26.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407–19. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 27.Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61:157–68. doi: 10.1093/cid/civ220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahal HS, Marseille EA, Tice JA, et al. Comparative clinical effectiveness and value of novel interferon-free combination therapy for hepatitis C genotype 1: Summary of California Technology Assessment Forum Report. JAMA Intern Med. 2015;175:1559–60. doi: 10.1001/jamainternmed.2015.3348. [DOI] [PubMed] [Google Scholar]
- 29.McCombs JS, Tonnu-MiHara I, Matsuda T, McGinnis J, Fox S. Can hepatitis C treatment be safely delayed? Evidence from the Veterans Administration Healthcare System. 2015 International Liver Congress; Vienna, Austria. April 22–26, 2015; p. Abstract O003. [Google Scholar]
- 30.Zahnd C, Salazar-Vizcaya LP, Dufour JF, et al. Impact of deferring HCV treatment on liver-related events in HIV+ patients. 2015 Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. February 23–24, 2015; p. Abstract 150. [Google Scholar]
- 31.Lo Re V, 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology. 2012;56:1688–98. doi: 10.1002/hep.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Re V, 3rd, Lynn K, Stumm ER, et al. Structural bone deficits in HIV/HCV-coinfected, HCV-monoinfected, and HIV-monoinfected women. J Infect Dis. 2015;212:924–33. doi: 10.1093/infdis/jiv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–6. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 34.Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. Am J Kidney Dis. 2011;57:396–402. doi: 10.1053/j.ajkd.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21:2269–80. doi: 10.3748/wjg.v21.i8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao JM, Fischer MA. Early Patterns of Sofosbuvir Utilization by State Medicaid Programs. N Engl J Med. 2015;373:1279–81. doi: 10.1056/NEJMc1506108. [DOI] [PubMed] [Google Scholar]
- 38.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Medicare & Medicaid Services. [Accessed on November 14, 2015];Medicaid Drug Rebate Notice: Assuring Medicaid Beneficiaries Access to Hepatitis C (HCV) Drugs. 2015 Nov 5; Accessed at: http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Downloads/Rx-Releases/State-Releases/state-rel-172.pdf.