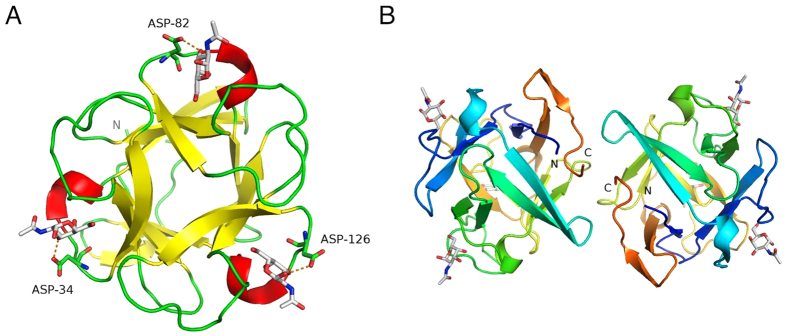
Figure 1. The overall structure of MytiLec.
(A) The Cα trace of one MytiLec subunit, looking along the pseudo-three-fold symmetry axis. The trace is coloured by secondary structure, with α-helices shown as red coils and β-strands as yellow arrows. α-GalNAc ligands are shown as sticks with white carbon atoms, and the conserved aspartic acid residue at each site is shown as sticks and labelled. The hydrogen bond between this aspartate and the ligand is shown as a dashed line. Secondary structure was determined automatically by PYMOL46. (B) The MytiLec dimer, viewed down the dyad axis. Each subunit is coloured from blue (N-terminus) to orange (C-terminus). α-GalNAc ligands are shown as sticks, with carbon atoms white and oxygen atoms red.