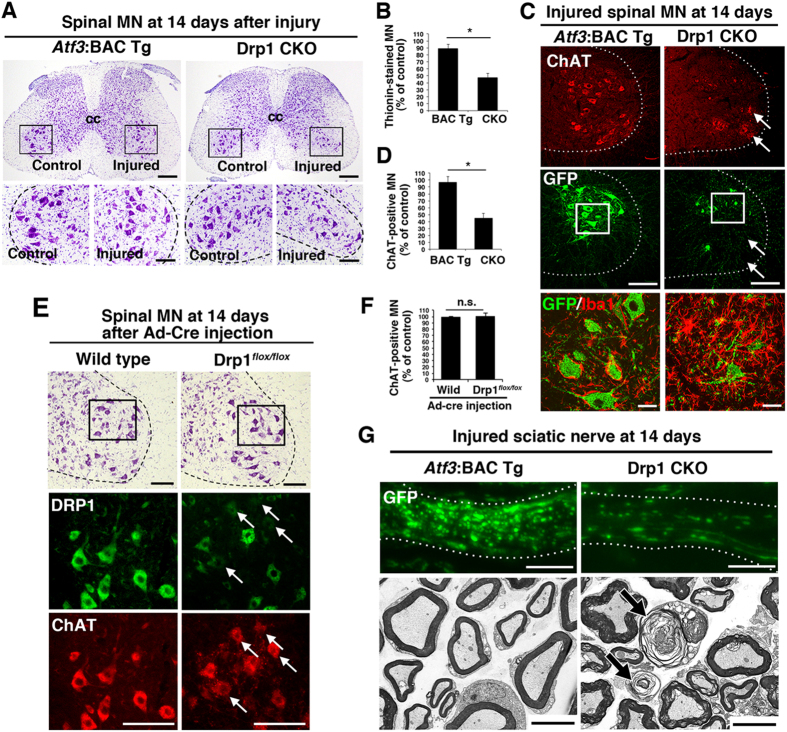
Figure 7. Injury-induced prevention of mitochondrial fission leads to neuronal death and axonal degeneration at 14 days after nerve injury.
(A) Thionin staining of spinal cord sections in Atf3:BAC Tg and Drp1 CKO mice at 14 days after sciatic nerve injury. The area surrounded by a black box in the upper panel is magnified in the lower panel. (B) Percentage of thionin-stained surviving motor neurons at 14 days after sciatic nerve injury. Results represent the percent ratio of surviving motor neurons on the injured side compared with the contralateral side. Data are the mean ± SEM. n = 8 mice per group, *p < 0.001. (C) Immunostaining of injured spinal motor neurons for ChAT (red) and GFP (green). Note the presence of GFP-negative motor neurons (arrows). The area surrounded by a box is magnified in lowest panel showing that microglial activation (Iba1, red) in the proximity of injured motor neurons (GFP, green). (D) Percentage of ChAT-positive surviving motor neurons on the injured side compared with the control side at 14 days after sciatic nerve injury. Data are the mean ± SEM. n = 8 mice per group, *p < 0.001. (E) Thionin staining of spinal motor neurons at 14 days after injection of adenovirus carrying cre recombinase (Ad-Cre) in wild-type and Drp1flox/flox mice (highest panel). The area surrounded by a box in highest panel was shown by immunostaining of DRP1 and ChAT in lower panels. Arrows indicate the reduced expression of DRP1 in ChAT-positive motor neurons. (F) Percentage of ChAT-positive motor neurons in wild-type and Drp1flox/flox mice at 14 days after injection of Ad-Cre. Data are means ± SEM. n = 4 mice per group, *p < 0.001. (G) Immunohistochemical staining of GFP (upper) and electron micrograph using x 3,000 objective of injured sciatic nerve (lower) in Atf3:BAC Tg and Drp1 CKO mice at 14 days after nerve injury. Arrows indicate degenerating axons. BAC Tg; Atf3:BAC Tg mice, CKO; Drp1 conditional knockout mice, Scale bars, 250 μm in (A, upper), 100 μm in (A, lower; C, middle; E), 30 μm in (C, lowest) and 5 μm in (G).