Abstract
Fabry disease is a rare X-linked lysosomal storage disease characterized by the dysfunction of multiple systems, including significant gastrointestinal involvement such as diarrhea, abdominal pain, early satiety and nausea. The gastrointestinal symptoms of Fabry disease are thought to be due to neuropathic and myopathic changes leading to symptoms of dysmotility that are encountered in many other disorders. The gastrointestinal symptoms can often be one of the presenting signs of the disease in childhood, but can be misdiagnosed by gastroenterologists for many years due to their nonspecific presentation. As the chief treatment for Fabry is enzyme-replacement therapy that has been shown to stabilize and possibly reverse disease course, recognition of these symptoms and early diagnosis in an attempt to prevent progression with treatment, is critical.
Keywords: abdominal pain, diarrhea, lysosomal storage disease, neuropathy
Introduction
Fabry disease is an X-linked lysosomal storage disease characterized by the dysfunction of multiple systems, including significant gastrointestinal (GI) involvement such as diarrhea, abdominal pain, early satiety and nausea. Although Fabry disease was thought to be rare, only affecting 1 in 40,000 [Meikle et al. 1999], recent newborn screening data show it to be much more common, affecting up to 1 in 3400–4000 newborns [Scott et al. 2013]. The specific GI symptoms are similar to those encountered in various disease presentations, leading to frequent delay in diagnosis and mistreatment. This review article is intended to provide detailed and updated Fabry information for gastroenterologists, thereby promoting broader recognition of the disease and its GI manifestations; thus, in turn, leading to more accurate and timely diagnosis followed by early initiation of disease-altering therapy.
Epidemiology
Fabry disease is the second most common lysosomal storage disease, defined by a deficiency of alpha-galactosidase, leading to the accumulation of a glycolipid, globotriaosylceramide (GL3), and its metabolites within many types of cells, including endothelial, cardiac and neuronal. The disease characteristically presents with acroparesthesias, neuropathic pain in the hands and feet worsened by temperature changes, and angiokeratomas, cutaneous manifestations frequently noted on the chest, groin and back [Zarate and Hopkin, 2008]. Without treatment, patients typically succumb to the disease up to 20 years prematurely, secondary to proteinuric renal failure, stroke or cardiac disease (arrhythmias, cardiomyopathy, coronary artery disease) [MacDermot et al. 2001].
As Fabry is an X-linked disease, females were previously considered heterozygous carriers without disease presentation, however, more recent studies demonstrate females can frequently experience variable and severe disease manifestations, similar to male hemizygotes. Female symptoms are thought, in part, to be secondary to X-inactivation in addition to other undetermined factors of penetrance [Deegan et al. 2006]. Thus, although some females, at times, have greater enzyme activity, a significant number of them develop symptoms with the same severity as males without correlation to enzyme level.
The GI symptoms are some of the most frequent and early general complaints among Fabry patients. Since the initial GI case study by Van Wayjen in 1958 [Van Wayjen, 1958], studies have repeatedly found many Fabry patients suffering from GI symptoms. Hoffmann and colleagues published results from the European Fabry Outcome Survey with 714 patients and found 52% of adults had GI complaints [Hoffmann et al. 2007]. Other large cohort studies had similar findings with GI symptoms affecting 16–70% of patients [Eng et al. 2007]. They also found that females initially have milder disease with eventual equal symptom severity over time and slightly higher incidence of GI manifestations [Deegan et al. 2006].
Significant GI involvement has also been observed in children and can progress in severity with age. Studies note prevalence rates in the range 18–60%, with boys tending to have increased severity and earlier presentation than girls [Ries et al. 2003; Hopkin et al. 2008]. These GI ailments are seen early in childhood, with a median onset age of 5 years in boys and 9.5 years in girls. In a systematic review, Laney and colleagues found that GI symptoms even presented in very young children, aged 1–4 years old, most commonly complaining of abdominal pain [Laney et al. 2015]. The symptoms present soon after the development of acroparesthesias and can be the initial symptom of Fabry disease in up to 20% of patients [Ries et al. 2003].
Gastrointestinal clinical phenotype
The GI symptoms occur anywhere along the GI tract varying in intensity and frequency and include a wide range of symptoms including abdominal pain, bloating, nausea, constipation and diarrhea. Patient presentation can vary from experiencing one severe symptom to a combination of multiple symptoms affecting daily functioning and health.
The most common complaint and often the initial GI symptom is abdominal pain affecting up to one third of patients [Hoffmann et al. 2007]. Patients describe cramping mid-abdominal discomfort, frequently worsened with meals and increased stress. Anecdotally, patients report severe, debilitating pain, commonly within several minutes of eating with changes in diet and frequency of meals altering the pain severity and duration.
The second most common GI symptom is diarrhea occurring in 20% of patients [Hoffmann et al. 2007]. The diarrhea can be intake-triggered, frequently associated with significant urgency and frequency, and occurring up to 15 times daily. Some patients report such severe urgency leading to routine fecal incontinence. Of note, unlike other inflammatory processes, patients do not have blood or mucous in their stool.
Although diarrhea is a common complaint, there is a subset of patients, mostly female, who experience debilitating constipation [Deegan et al. 2006]. Additionally, some Fabry patients describe a cyclical pattern of alternating diarrhea and constipation interspersed with periods of quiescence and normal bowel movements, making diagnosis and management particularly difficult [Keshav, 2006].
Somewhat less common, but significant, are reports of upper GI symptoms including nausea, vomiting and early satiety that lead to severe diet restriction and fear of ingestion of nutrients. In a series of gastric emptying scans, Argoff and colleagues found that five out of seven patients with GI complaints had significantly delayed gastric emptying [Argoff et al. 1998].
Additionally, initial papers cited malnutrition and lower body mass index (BMI) among patients presumed as caused by anorexia secondary to abdominal discomfort [MacDermot et al. 2001], but these findings have not been confirmed by more recent studies [Hoffmann et al. 2007]. Hopkin and colleagues’ pediatric study did find a lower BMI in Fabry boys as compared with healthy boys that was not similarly noted in girls, thought to be due to earlier disease onset and higher disease burden in boys [Hopkin et al. 2008]. However, a systematic review of young children with Fabry found that early growth was normal in both boys and girls, suggesting that growth problems from Fabry are not specifically present in early childhood [Laney et al. 2015].
Case reports have also identified more severe and localized disease manifestations including cholelithiasis, achalasia, and autoimmune diseases such as celiac and Crohn’s disease. Although rare, reports of complications requiring significant interventions include jejunal diverticulosis, leading to perforation, pseudo-obstruction, fistulas, and bowel ischemia, have been described [Buda et al. 2013; Politei et al. 2015]. Some have suggested a GI phenotype of Fabry disease, consisting of more severe and intense GI symptoms with an earlier onset than the other classical symptoms of disease [Buda et al. 2012].
Adult patients with GI symptoms had significantly lower quality of life (EQ-5D) score than patients without GI symptoms, particularly among those with diarrhea [Hoffmann et al. 2007]. Quality-of-life surveys completed in children with Fabry show lower scores in comparison with healthy subjects, especially in those with acroparesthesias and GI symptoms [Hopkin et al. 2008], with many patients likely missing school due to various GI complaints. Although children characteristically experience less disease burden than that in adults, including a typical absence of significant renal and cardiac involvement, it is clear that the GI symptoms that do present in childhood have a considerable negative impact on daily functioning. Thus, emphasis is on the need for adequate and appropriate diagnosis and intervention.
Pathophysiology
A hallmark of Fabry disease is the systemic glycolipid accumulation, which has been supported by various histopathology studies, including renal, cardiac and dermal biopsies [Breunig et al. 2003; Askari et al. 2007]. Accumulation is thought to start prenatally with GL3 found in the placenta and fetal tissue including renal and cardiac cells in males [Elleder et al. 1998; Thurberg and Politei, 2012]. Both the neuronal and vascular dysfunction caused by the physical GL3 accumulation, in addition to the disruption of cellular signaling, leads to ischemia and inflammation with resulting effects on multiple systems, including the autonomic nervous system (ANS) and GI tract [Zarate and Hopkin, 2008].
Vasculopathy
Vascular abnormalities can cause cell injury via both mechanical and signaling pathways. GL3 accumulates in the endothelium and smooth muscle cells leading to vessel wall expansion [Namdar et al. 2012]. Additionally, the deposition alters cellular signaling pathways, inducing extra cellular matrix proliferation of the smooth muscle cell leading to hypertrophy, myopathy, and vascular remodeling [Boutouyrie et al. 2002; Rombach et al. 2010]. The combination of these processes produces significant intraluminal thickening, with subsequent decreased vessel flow, ultimately causing ischemia, infarction and eventual end-organ damage [Sheth et al. 1981]. The microvasculature is affected earlier and more severely than the macrovasculature [Boutouyrie et al. 2002]. This ischemic effect on the abdominal and mesenteric vasculature, including neuronal, is suspected to be a source of many of the GI symptoms.
Neuropathy
Patients with Fabry disease have dysregulation of the ANS, producing systemic symptoms such as abnormal sweating, reduced saliva and dysmotility [Hilz, 2002; Burlina et al. 2011]. This ANS neuropathy is secondary to both neuronal glycosphingolipid accumulation and ischemic injury.
Flynn and colleagues in 1972 confirmed neuronal accumulation in the GI tract, reporting enlarged and vacuolated neurons in the Meissner plexus of the jejunum and rectum [Flynn et al. 1972]. Similar follow-up studies of the bowel have comparable findings with cytoplasmic deposition of presumed GL3 within various neuronal structures [O’Brien et al. 1982], thus suggesting that accumulation of GL3 affects the GI neuronal cells similarly to other organ systems.
In addition to glycolipid accumulation, vascular occlusion of the vasa vasorum of the peripheral nerves via mechanisms previously mentioned leads to neuronal ischemia and dysregulation [Hilz, 2002]. The dorsal root ganglia have the finest associated blood vessels and thus are particularly susceptible to injury from these vascular changes [Keshav, 2006]. Additionally, studies show preferential disruption in the small, thin, unmyelinated nerve fibers associated with peripheral pain perception and the enteric nervous system, with relative sparing of the thick myelinated nerve fibers [Dutsch et al. 2002].
Inflammation
Although accumulation and ischemia are hypothesized to be the key contributors to the disease manifestation, newer studies have discovered glycolipid’s additional effect on cellular functioning and protein expression leading to a pro-inflammatory, prothrombotic state. For example, GL3 deposition was found to cause alterations in the invariant natural killer T cells [Pereira et al. 2013], leading to an amplified inflammatory response. Additionally, in mouse models, deposition generated interference with the nitric oxide pathways inducing a prothrombotic environment [Park et al. 2009]. Specifically, when examining the mesenteric microvasculature of the GI tract in a Fabry mouse model, Kang and colleagues found endothelial dysfunction with reduction in vasodilatory capabilities that was associated with decreased nitric oxide bioavailability [Kang et al. 2014]. The presentation of many symptoms in childhood indicates that even small amounts of accumulation can cause underlying damage and calls for the continued exploration of the specific effect of GL3 accumulation on the cellular signaling and functioning.
Clinical correlation
These pathways of dysfunction can be translated clinically to further understand the etiology and mechanisms of symptom presentation. For example, it is postulated that abdominal pain is neuroischemic in nature due to inadequate blood flow to the GI tract, supported by the amplification of pain intensity with increased metabolic demand. Additionally, small-fiber neuropathy, similar to peripheral neuropathy-causing acroparesthesias likely contributes to the abdominal pain.
The diarrheal symptoms are hypothesized to be due to a combination of underlying disease processes including ANS abnormalities, such as the derangement of the myenteric autonomic plexus, causing hyperactive uncoordinated contractions. Furthermore, a component of the diarrhea may be due to GL3 accumulation within the villi, leading to inflammation, decreased villi activity and resulting malabsorption. Additionally, small bowel microbial overgrowth, as demonstrated by O’Brien and colleagues in jejunal aspirate from Fabry patients [O’Brien et al. 1982], can contribute. The overgrowth is likely secondary to neuropathic-reduced peristalsis and eventual stasis, leading to a favorable environment for bacterial accumulation and resulting diarrhea.
The upper GI symptoms are thought to be due to substrate accumulation causing neuronal dysfunction, supported by O’Brien and colleagues where they noted increased sphingolipid storage in ganglion cells of the ANS in those patients with delayed gastric emptying [O’Brien et al. 1982]. Additionally, the autonomic small-fiber damage noted in Fabry disease is similar to that seen in diabetic neuropathy, therefore suggesting that GI symptoms found in patients with Fabry disease may have a similar mechanism to the gastropathy seen in diabetes [Buda et al. 2013].
The more severe and life-threatening complications, including diverticulitis and perforation, are also likely due to underlying dysmotility. The neuronal dysfunction leading to hyperactivity and lack of muscular coordination results in weakening of the muscle wall; thus, allowing for areas of out-pocketing of weakened muscle and diverticulosis, increases the risk of infection and perforation [Politei et al. 2015].
Differential diagnosis
As many of these GI symptoms can be nonspecific, especially those that present early in life, and additional unique characteristics of Fabry disease do not present until the patient reaches adulthood, many children, adolescents and adults will be misdiagnosed. When there is no evidence or suspicion of Fabry disease in the family, patients can carry other diagnoses including Crohn’s disease, celiac, or irritable bowel syndrome (IBS), prior to receiving the correct diagnosis, as many healthcare providers are unfamiliar with the disease. Marchesoni and colleagues followed 45 consecutive patients with Fabry and found that 10 pediatric patients presenting with abdominal pain had been diagnosed with food intoxication or nonspecific abdominal pain prior to eventual Fabry diagnosis [Marchesoni et al. 2010]. Cross-sectional population studies have found a 10–19 years’ delay in diagnosis from initial presentation of symptoms, with mean age of diagnosis at 28-years old [Pintos Morell, 2002; Cimaz et al. 2011]. Hence, there is critical necessity of increased awareness and knowledge about the signs and symptoms of the disease in the medical community, particularly gastroenterologists, ensuring prompt diagnosis and treatment.
The GI symptoms in Fabry disease can be seen in a wide variety of disease processes including other lysosomal storage diseases such as Gaucher disease, and other systemic diseases, such as mitochondrial disease, glycogen-storage diseases and scleroderma. More common illnesses of motility such as IBS-diarrhea type (IBS-D), have many overlapping symptoms with Fabry patients with frequent episodes of nonbloody diarrhea and abdominal pain. Pensabene and colleagues found that out of 33 Fabry patients with GI symptoms, 64% of adults and 25% of children also met criteria for a functional GI disorder, making diagnosis even more difficult [Pensabene et al. 2015].
Although Fabry disease is relatively uncommon, it should be considered in the differential diagnosis of patients presenting with symptoms of prolonged GI dysmotility with multiple organ dysfunction, particularly neuronal and cutaneous symptoms or with abnormal evaluation findings including proteinuria or arrhythmia on EKG. Specifically, GI symptoms that are frequently associated with Fabry disease presentation include: severe postprandial abdominal pain, noninflammatory diarrhea associated with significant urgency, and early satiety or gastroparesis; such symptoms should arouse suspicion in a gastroenterologists. Additionally, physicians should be alerted to the disease if a patient complains of symptoms related to ANS dysfunction, including neuropathic pain in the extremities, heat intolerance or abnormal sweating. Finally, a family history of multiple relatives with significant renal, cardiac or neuronal disease would warrant additional evaluation for a genetic cause to the GI presentation.
Evaluation
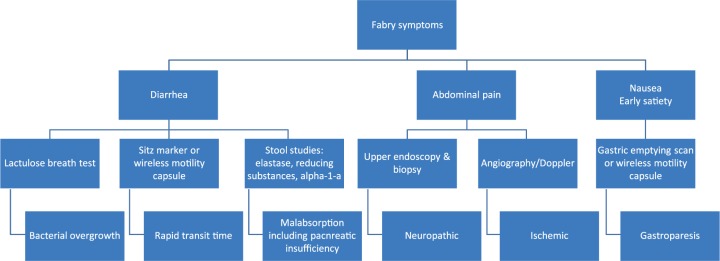
The diagnosis of Fabry disease is made by measurement of alpha-galactosidase A activity in blood leukocytes, whole blood (dried blood spot), or in the tissues. In heterozygous females, with often low-normal levels of enzyme activity, molecular testing is required for definitive diagnosis. There are multiple modalities used for diagnosis and differentiation of the Fabry GI symptoms from other common GI diseases including radiologic, and histologic studies (Figure 1).
Figure 1.
Workup of common symptoms of Fabry disease.
Radiologic studies typically focus on blood flow and gut motility. In those with signs of ischemic-like symptoms such as abdominal pain, a Doppler scan with mesenteric blood flow or angiography can be obtained to assess underlying vascular compromise. A gastric emptying scan should be obtained to analyze upper tract motility in those with upper GI symptoms such as nausea and early satiety, while a Sitz mark test can be utilized to examine colonic transit time in those with abnormal bowel movements. Additionally, a barium enema can look for haustra reduction [O’Brien et al. 1982; Politei et al. 2015].
Visualization of the intestine with colonoscopies and endoscopies can be utilized to search for signs of mucosal damage or inflammation, however, are typically macroscopically normal. Superficial biopsies taken from the bowel examined under light microscopy will likely reveal normal villous architecture and surface epithelium but may show enlarged and vacuolated neurons with GL3 deposition [O’Brien et al. 1982; Jack et al. 1991]. The blood vessels and muscle cells of the muscularis mucosa may have deposits with luxol fast blue positive stain. Electron microscopy can display electron-dense intralysosomal striped ‘zebra-like’ bodies in smooth muscle and ganglion cells, consistent with a deposition disorder [Simon et al. 1990]. Renal, cardiac and dermal specimens have previously shown neuronal swelling, demyelination, vascular sclerosis, intimal fibrosis and hypertrophy of the smooth muscles of the arterioles [Valbuena et al. 2008] that at this point, has not been studied extensively along the GI tract. It is hypothesized, though, that once more in-depth analysis is completed of GI specimens, findings similar to those found in other organ systems would be encountered, supporting the likely symptom etiology of neuronal abnormalities and dysmotility.
Blood work including serum protein, albumin, folate, vitamin B12, calcium and phosphate levels are typically normal. Therefore, if a patient has significantly abnormal levels, other diagnoses should be considered. Recent studies have noted anemia to be a feature of Fabry patients, likely due to underlying renal and cardiac issues; however, GI causes should be ruled out [Kleinert et al. 2005]. Additionally, as heat can worsen Fabry symptoms, patients frequently avoid the sun, thus leading to low vitamin D levels.
Pediatric patients can present a more complex diagnostic challenge, as there is more concern for radiation exposure, repeated blood draws and anesthesia required for endoscopic procedures. Therefore, diagnostic GI studies should be reserved for those who have been unresponsive to empiric treatment, or in which further studies will alter management or provide additional information about the disease process.
Management
With improved natural history data, Fabry disease’s course is better understood and with the advent of enzyme-replacement therapy (ERT), more emphasis has been placed on targeting the underlying disease process in addition to focused management of specific symptoms (Table 1).
Table 1.
Pharmacologic therapies for Fabry gastrointestinal symptomatology.
| Symptom | Etiology | Medication |
|---|---|---|
| Diarrhea | Bacterial overgrowth | Rifaxamin, probiotic, tetracycline |
| Rapid transit | Lomotil, tincture of opium | |
| Malabsorption | Dietary changes | |
| Pancreatic | Pancreatic enzyme replacement | |
| Abdominal pain | Neuropathic | Amitriptyline, carbemazapine, pregabalin, Gabapentin |
| Gastric inflammation | Proton pump inhibitors, H2 blockers | |
| Nausea, early satiety | Gastroparesis | Metoclopramide, erythromycin, ondansetron |
Several medical interventions have been trialed to target the various GI symptoms. Argoff and colleagues studied gastroparetic patients and found significant improvement in their symptoms with metoclopramide treatment [Argoff et al. 1998]. As the gastroparesis is likely due to neuronal dysfunction similar to diabetic gastropathy, metoclopramide is suspected to work in a similar manner. Therefore, in patients with nausea, vomiting or early satiety, promotility drugs should be initiated.
Both carbamazepine and gabapentin are shown to be effective analgesics in patients with autonomic dysfunction and chronic peripheral neuropathic pain [Burlina et al. 2011]. Filling-Katz and colleagues found that five out of seven Fabry patients treated with carbamazepine had improved peripheral neuropathy [Filling-Katz et al. 1989]. Therefore, these neuromodulators may conceivably be of benefit in the treatment of neuropathic abdominal pain as well and thus, should be trialed in patients.
Encouraging responses were seen with the use of other symptomatic-alleviating medications. However, due to small numbers and lack of validation, it is unclear what the actual utility of these treatments is. For example, anecdotally, ondansetron administration can improve nausea, while supplementing with pancreatic enzymes and H2 blockers relieved several GI symptoms. Tetracycline for bacterial overgrowth and loperimde to decrease hyperactive contractions in patients with diarrhea can be used. Probiotics and dietary alterations, including specific food elimination, changing in timing of meals, and quantity of intake have also exhibited promising results. In severe GI complications, such as obstruction or perforation, surgery can be performed [Eng et al. 2007]. As pediatric patients typically present with abdominal pain or diarrhea, an empiric trial of antidiarrhea medication, promotility or neuromodulation might be warranted prior to invasive procedures.
Since the advent of ERT, emphasis has been placed on treating the underlying dysfunction as early as possible. ERT was introduced in 2001 in Europe with agalsidase alpha (Replagal: Shire, Jersey, France) and 2003 in the United States with agalsidase beta (Fabrazyme: Genzyme, a Sanofi Company, Cambridge, MA), both acting as replacement enzymes of alpha-galactosidase.
Since its initiation, multiple studies have shown that introduction of ERT can improve patients’ overall health status, including GI symptoms. The enzyme replacement leads to greater clearance of GL3, as demonstrated in studies in which patients with elevated plasma GL3 treated with ERT had a decrease in serum levels [Schiffmann et al. 2014]. This prevention of accumulation likely leads to improved mesenteric flow, inhibiting both ischemia and neuronal-cell signaling disruption. Hoffmann and colleagues found that patients treated with 24 months of ERT had a reduction in their overall GI symptoms, particularly abdominal pain [Hoffmann et al. 2007]. Ramaswami followed almost 100 children after 1 year of ERT treatment and found a decrease in GI symptoms in boys, including constipation, abdominal pain and diarrhea [Ramaswami, 2008]. Unfortunately, in many of these studies, although improvement is noted, more than half of the patients continue to have GI symptoms after a significant amount of ERT intervention. Some patients even develop new GI symptoms while on ERT, thus it remains a significant cause of morbidity [Hoffmann et al. 2004], necessitating concurrent, targeted, GI-specific treatment.
Recently, ERT has been shown to be effective in stabilizing progression and even reversing mild to moderate kidney involvement [Tondel et al. 2013]. The effects of ERT on the GI symptoms have shown promising results, indicating that some GI manifestation might be reversible in at least a subset of patients with Fabry disease. At this time, there are no specific guidelines for optimal time of initiation of ERT. However, as it is known that accumulation starts in utero and ERT treatment can prevent progression, there is significant emphasis on early initiation of ERT.
Conclusion
Additional studies and longer-term follow up may help characterize the GI respondents, elucidate their underlying pathology and further inform about appropriate age of initiation. Particularly, future research direction should focus on clarifying the underlying dysmotility and further analyzing the correlation between the dysmotility, the histologic disease progression and the clinical symptom presentation in order to gain a broad understanding of the GI disease burden and future targeted goals of therapy. Furthermore, a validated assessment scale of GI symptoms is needed in order to improve clinical evaluation and comparison.
GI manifestations of Fabry disease are frequent and can be extremely debilitating. By identifying those with Fabry disease, not only can appropriate interventions be introduced, but additional family members can be screened and diagnosed correctly. Although Fabry disease is rare, the GI manifestations are those that are encountered frequently, both in common illnesses and other rare storage diseases, therefore complicating prompt diagnosis. As recent studies show that prolonged treatment with ERT can lead to stabilization and possible reversal of disease, and that early treatment may produce improved outcomes, there is incentive for clinicians to be prompt in their diagnosis of Fabry disease. With broader recognition of the GI symptoms, patients can receive earlier diagnosis with initiation of appropriate treatment, thus improving their medical care, quality of life and possibly the lives of those in their extended family.
Acknowledgments
There are no additional acknowledgements of financial, material or author support.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Claire Zar-Kessler, Katherine Bustin Sims and Braden Kuo participated in a Fabry training fellowship supported by an unrestricted grant from Genzyme.
Contributor Information
Claire Zar-Kessler, MGH Center for Neurointestinal Health, Division of Pediatric Gastroenterology, Hepatology and Nutrition, Massachusetts General Hospital for Children, 175 Cambridge St CPZ-575, Boston, MA 02114, USA.
Amel Karaa, Genetics Unit, Massachusetts General Hospital, Boston, MA, USA.
Katherine Bustin Sims, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA.
Virginia Clarke, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA.
Braden Kuo, MGH Center for Neurointestinal Health, GI Unit Massachusetts General Hospital, Boston, MA, USA.
References
- Argoff C., Barton N., Brady R., Ziessman H. (1998) Gastrointestinal symptoms and delayed gastric emptying in Fabry’s disease: response to metoclopramide. Nucl Med Commun 19: 887–891. [DOI] [PubMed] [Google Scholar]
- Askari H., Kaneski C., Semino-Mora C., Desai P., Ang A., Kleiner D., et al. (2007) Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch 451: 823–834. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P., Laurent S., Laloux B., Lidove O., Grunfeld J., Germain D. (2002) Arterial remodelling in Fabry disease. Acta Paediatr 91: 62–66. [DOI] [PubMed] [Google Scholar]
- Breunig F., Weidemann F., Beer M., Eggert A., Krane V., Spindler M., et al. (2003) Fabry disease: diagnosis and treatment. Kidney Int 84: S181–S185. [DOI] [PubMed] [Google Scholar]
- Buda P., Ksiazyk J., Tylki-Szymanska A. (2013) Gastroenterological complications of Anderson-Fabry disease. Curr Pharm Des 19: 6009–6013. [DOI] [PubMed] [Google Scholar]
- Buda P., Wieteska-Klimczak A., Ksiazyk J., Gietka P., Smorczewska-Kiljan A., Pronicki M., et al. (2012) Gastrointestinal phenotype of Fabry disease in a patient with pseudoobstruction syndrome. JIMD Rep 4: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlina A., Sims K., Politei J., Bennett G., Baron R., Sommer C., et al. (2011) Early diagnosis of peripheral nervous system involvement in Fabry disease and treatment of neuropathic pain: the report of an expert panel. BMC Neurol 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimaz R., Guillaume S., Hilz M., Horneff G., Manger B., Thorne J., et al. (2011) Awareness of Fabry disease among rheumatologists–current status and perspectives. Clin Rheumatol 30: 467–475. [DOI] [PubMed] [Google Scholar]
- Deegan P., Baehner A., Barba Romero M., Hughes D., Kampmann C., Beck M., et al. (2006) Natural history of Fabry disease in females in the Fabry outcome survey. J Med Genet 43: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutsch M., Marthol H., Stemper B., Brys M., Haendl T., Hilz M. (2002) Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol 19: 575–586. [DOI] [PubMed] [Google Scholar]
- Elleder M., Poupetova H., Kozich V. (1998) [Fetal pathology in Fabry’s disease and mucopolysaccharidosis type I]. Cesk Patol 34: 7–12. [PubMed] [Google Scholar]
- Eng C., Fletcher J., Wilcox W., Waldek S., Scott C., Sillence D., et al. (2007) Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry registry. J Inherit Metab Dis 30: 184–192. [DOI] [PubMed] [Google Scholar]
- Filling-Katz M., Merrick H., Fink J., Miles R., Sokol J., Barton N. (1989) Carbamazepine in Fabry’s disease: effective analgesia with dose-dependent exacerbation of autonomic dysfunction. Neurology 39: 598–600. [DOI] [PubMed] [Google Scholar]
- Flynn D., Lake B., Boothby C., Young E. (1972) Gut lesions in Fabry’s disease without a rash. Arch Dis Child 47: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz M. (2002) Evaluation of peripheral and autonomic nerve function in Fabry disease. Acta Paediatr Suppl 91: 38–42. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Reinhardt D., Koletzko B. (2004) Effect of enzyme-replacement therapy on gastrointestinal symptoms in Fabry disease. Eur J Gastroenterol Hepatol 16: 1067–1069. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Schwarz M., Mehta A., Keshav S: Fabry Outcome Survey European Investigators. (2007) Gastrointestinal symptoms in 342 patients with Fabry disease: prevalence and response to enzyme replacement therapy. Clin Gastroenterol Hepatol 5: 1447–1453. [DOI] [PubMed] [Google Scholar]
- Hopkin R., Bissler J., Banikazemi M., Clarke L., Eng C., Germain D., et al. (2008) Characterization of Fabry disease in 352 pediatric patients in the Fabry registry. Pediatr Res 64: 550–555. [DOI] [PubMed] [Google Scholar]
- Jack C., Morris A., Nasmyth D., Carroll N. (1991) Colonic involvement in Fabry’s disease. Postgrad Med J 67: 584–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Shu L., Park J., Shayman J., Bodary P. (2014) Endothelial nitric oxide synthase uncoupling and microvascular dysfunction in the mesentery of mice deficient in alpha-galactosidase A. Am J Physiol Gastrointest Liver Physiol 306: G140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshav S. (2006) Gastrointestinal manifestations of Fabry Disease. In: Mehta A., Beck M., Sunder-Plassmann G. (eds), Fabry disease: perspectives from 5 years of FOS. Oxford: Oxford PharmaGenesis. [PubMed] [Google Scholar]
- Kleinert J., Dehout F., Schwarting A., De Lorenzo A., Ricci R., Kampmann C., et al. (2005) Anemia is a new complication in Fabry disease: data from the Fabry Outcome Survey. Kidney Int 67: 1955–1960. [DOI] [PubMed] [Google Scholar]
- Laney D., Peck D., Atherton A., Manwaring L., Christensen K., Shankar S., et al. (2015) Fabry disease in infancy and early childhood: a systematic literature review. Genet Med 17: 323–330. [DOI] [PubMed] [Google Scholar]
- MacDermot K., Holmes A., Miners A. (2001) Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 38: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesoni C., Roa N., Pardal A., Neumann P., Caceres G., Martinez P., et al. (2010) Misdiagnosis in Fabry disease. J Pediatr 156: 828–831. [DOI] [PubMed] [Google Scholar]
- Meikle P., Hopwood J., Clague A.E, Carey W. (1999) Prevalence of lysosomal storage disorders. JAMA 281: 249–254. [DOI] [PubMed] [Google Scholar]
- Namdar M., Gebhard C., Studiger R., Shi Y., Mocharla P., Schmied C., et al. (2012) Globotriaosylsphingosine accumulation and not alpha-galactosidase-a deficiency causes endothelial dysfunction in Fabry disease. PLoS One 7: e36373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien B., Shnitka T., Mcdougall R., Walker K., Costopoulos L., Lentle B., et al. (1982) Pathophysiologic and ultrastructural basis for intestinal symptoms in Fabry’s disease. Gastroenterology 82: 957–962. [PubMed] [Google Scholar]
- Park J., Shu L., Shayman J. (2009) Differential involvement of COX1 and COX2 in the vasculopathy associated with the alpha-galactosidase a-knockout mouse. Am J Physiol Heart Circ Physiol 296:H1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensabene L., Sestito S., Nicoletti A., Graziano F., Strisciuglio P., Concolino D. (2015) Gastrointestinal symptoms of patients with Fabry disease. Gastroenterology Research and Practice 2016: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C., Azevedo O., Maia M., Dias A., Sa-Miranda C., Macedo M. (2013) Invariant natural killer T cells are phenotypically and functionally altered in Fabry disease. Mol Genet Metab 108: 241–248. [DOI] [PubMed] [Google Scholar]
- Pintos Morell G. (2002) [Fabry’s disease: diagnosis in the pediatric age group]. An Esp Pediatr 57: 45–50. [PubMed] [Google Scholar]
- Politei J., Thurberg B., Wallace E., Warnock D., Serebrinsky G., Durand C., et al. (2015) Gastrointestinal involvement in Fabry disease. so important, yet often neglected. Clin Genet: [DOI] [PubMed] [Google Scholar]
- Ramaswami U. (2008) Fabry disease during childhood: clinical manifestations and treatment with agalsidase alfa. Acta Paediatr Suppl 97: 38–40. [DOI] [PubMed] [Google Scholar]
- Ries M., Ramaswami U., Parini R., Lindblad B., Whybra C., Willers I., et al. (2003) The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr 162: 767–772. [DOI] [PubMed] [Google Scholar]
- Rombach S., Twickler T., Aerts J., Linthorst G., Wijburg F., Hollak C. (2010) Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab 99: 99–108. [DOI] [PubMed] [Google Scholar]
- Schiffmann R., Pastores G., Lien Y., Castaneda V., Chang P., Martin R., et al. (2014) Agalsidase alfa in pediatric patients with Fabry disease: a 6.5-year open-label follow-up study. Orphanet J Rare Dis 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C., Elliott S., Buroker N., Thomas L., Keutzer J., Glass M., et al. (2013) Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-i from newborn blood spots by tandem mass spectrometry. J Pediatr 163: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth K., Werlin S., Freeman M., Hodach A. (1981) Gastrointestinal structure and function in Fabry’s disease. Am J Gastroenterol 76: 246–251. [PubMed] [Google Scholar]
- Simon M., Frey H., Gruler H., Bultmann B. (1990) Glycolipid storage material in Fabry’s disease: a study by electron microscopy, freeze-fracture, and digital image analysis. J Struct Biol 103: 40–47. [DOI] [PubMed] [Google Scholar]
- Thurberg B., Politei J. (2012) Histologic abnormalities of placental tissues in Fabry disease: a case report and review of the literature. Hum Pathol 43: 610–614. [DOI] [PubMed] [Google Scholar]
- Tondel C., Bostad L., Larsen K., Hirth A., Vikse B., Houge G., et al. (2013) Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 24: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena C., Carvalho E., Bustorff M., Ganhao M., Relvas S., Nogueira R., et al. (2008) Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch 453: 329–338. [DOI] [PubMed] [Google Scholar]
- Van Wayjen R. (1958) Enterocolitis as a symptom of angiokeratoma corporis diffusum. Ned Tijdschr Geneeskd 102: 1941–1943. [PubMed] [Google Scholar]
- Zarate Y., Hopkin R. (2008) Fabry’s disease. Lancet 372: 1427–1435. [DOI] [PubMed] [Google Scholar]