Abstract
Background
Capnography may provide useful non-invasive bedside information concerning heterogeneity in lung ventilation, ventilation–perfusion mismatching and metabolic status. Although the capnogram may be recorded by mainstream and sidestream techniques, the capnogram indices furnished by these approaches have not previously been compared systematically.
Methods
Simultaneous mainstream and sidestream time and volumetric capnography was performed in anaesthetized, mechanically ventilated patients undergoing elective heart surgery. Time capnography was used to assess the phase II (SII,T) and III slopes (SIII,T). The volumetric method was applied to estimate phase II (SII,V) and III slopes (SIII,V), together with the dead space values according to the Fowler (VDF), Bohr (VDB), and Enghoff (VDE) methods and the volume of CO2 eliminated per breath (). The partial pressure of end-tidal CO2 () was registered.
Results
Excellent correlation and good agreement were observed in SIII,T measured by the mainstream and sidestream techniques [ratio=1.05 (sem 0.16), R2=0.92, P<0.0001]. Although the sidestream technique significantly underestimated and overestimated SIII,V [1.32 (0.28), R2=0.93, P<0.0001], VDF, VDB, and VDE, the agreement between the mainstream and sidestream techniques in the difference between VDE and VDB, reflecting the intrapulmonary shunt, was excellent [0.97 (0.004), R2=0.92, P<0.0001]. The exhibited good correlation and mild differences between the mainstream and sidestream approaches [0.025 (0.005) kPa].
Conclusions
Sidestream capnography provides adequate quantitative bedside information about uneven alveolar emptying and ventilation–perfusion mismatching, because it allows reliable assessments of the phase III slope, and intrapulmonary shunt. Reliable measurement of volumetric parameters (phase II slope, dead spaces, and eliminated CO2 volumes) requires the application of a mainstream device.
Keywords: capnography, carbon dioxide, intraoperative monitoring, mechanical ventilation, ventilation-perfusion ratio
Editor's key points.
It is not clear which factors sidestream capnography can indicate as accurately as mainstream capnography.
Mainstream and sidestream time and volumetric capnography were performed to compare several factors in anaesthetized, mechanically ventilated patients undergoing elective heart surgery.
Sidestream capnography provides adequate quantitative bedside information about uneven alveolar emptying and ventilation–perfusion mismatch, but mainstream capnography is required for a reliable measurement of volumetric parameters.
Capnography is a non-invasive method for the numerical and graphical analysis of the exhaled CO2 concentration,1–5 and a valuable tool for the improvement of patient safety.6 Although assessment of capnogram shape factors is not yet a standard part of patient monitoring, it has the promise to provide routine information concerning pathophysiological processes of lung ventilation, such as airway patency7–10 and lung recoil tendency.8,9 Furthermore, combination of capnography with expired gas volume monitoring allows the assessment of ventilation–perfusion matching and the metabolic status of the body.3,5,10,11
In clinical practice, two techniques are available, based on the measurement site of CO2. Mainstream capnography applies an infrared sensor located proximally to the patient between the tracheal tube and the Y-piece, and thus, allows a rapid and accurate analysis of the CO2 concentration of the exhaled gas.12–14 However, this method is used mainly in intensive care units, because of the disadvantages posed by the local heating of the head and the weight of the sample cell, which increases the risk of tracheal tube dislocation.
As an alternative, sidestream capnography is often used in the operating theatre because it is easily manageable and allows the monitoring of other gases.7–9,15 These devices analyse the gas sample distally from the patient, and therefore, have the drawbacks of a prolonged total response time,16–18 the occurrence of axial mixing,2,10,11,19 and a variable suction flow rate.20 All these processes result in a dynamic distortion of the CO2 concentration curve, and thus, have a potential to bias the derived capnographic parameters.
There have been a few previous attempts to compare capnographic parameters obtained by sidestream and mainstream techniques, but they were the manufacturer's educational material,21 focused only on the end-tidal CO2 value in experimental22 and clinical studies,23–25 or were limited to a small cohort of infants.26 However, there is a lack of information about the relationship between capnographic indices obtained by sidestream and mainstream techniques in mechanically ventilated adults. Therefore, the aim of the present study was to validate the ability of the sidestream technique to provide adequate quantitative bedside information about uneven alveolar emptying and ventilation–perfusion mismatching. Therefore, we determined which of the capnogram parameters (shape factors, respiratory dead space) can be assessed reliably by applying the sidestream technique. We hypothesized that sidestream capnography is suitable to measure indices obtained from the quasi-static phases of the capnogram, whereas phases with transient CO2 concentration changes are exposed to measurement bias.
Methods
Patients
Twenty-nine patients [female/male: 13/16, 71 (57–85) yr old] undergoing elective cardiac surgery were enrolled into the study in a prospective consecutive manner. The study protocol was approved by the Human Research Ethics Committee of the University of Szeged, Hungary (no. WHO 2788). Written informed consent was obtained from each patient. Patients with severe cardiopulmonary disorders (pleural effusion >300 ml, ejection fraction <30%, BMI >35 kg m−2, or intraoperative acute asthma exacerbation) were excluded.
Anaesthesia and surgery
Anaesthesia was induced with i.v. midazolam (30 µg kg−1), sufentanil (0.4–0.5 µg kg−1), and propofol (0.3–0.5 µg kg−1), and was maintained by an i.v. propofol infusion (50 µg kg−1 min−1). Neuromuscular block was achieved by i.v. boluses of rocuronium (0.2 mg kg−1 every 30 min).
After tracheal intubation, the patients' lungs were mechanically ventilated in volume-controlled mode with descending flow (Dräger Zeus, Lübeck, Germany) by setting the tidal volume to 7 ml kg−1, the ventilator frequency to 9–14 bpm, and the PEEP to 4 cm H2O, and maintaining the inspired oxygen fraction at 0.5.
Recording and analyses of the expiratory capnogram
The measurement set-up was designed to allow the sampling of the mainstream (Capnogard®; Novametrix, Andover, MA, USA) and sidestream (Ultima™; Datex/Instrumentarium, Helsinki, Finland) capnographs from the same sampling site in the ventilator circuit. This was achieved by connecting the sampling port of the sidestream capnograph next to the mainstream sensor between the Y-piece and the tracheal tube. A screen pneumotachograph (Piston Ltd, Budapest, Hungary) was used to record the central airflow at the same point of the ventilator circuit. Simultaneous 15 s recordings of the CO2 signals of the mainstream and sidestream capnographs and the ventilation flow were digitized (sampling frequency 102.4 Hz) and analysed with custom-made software. Volumetric capnograms were constructed from the time capnograms and the integrated flow data. To compensate for the transport delay caused by the suction of the gas into the sample cell, the sidestream time capnograms were shifted by −1.65 s. This value was determined by analysing the time delay between the mainstream and sidestream capnogram curves during stepwise changes in CO2 concentration, in a similar manner to an earlier approach.17
The slopes of phase III of the time and volumetric capnograms determined by mainstream (SIII,T,MS and SIII,V,MS) and sidestream (SIII,T,SS and SIII,V,SS) capnography were assessed by fitting a linear regression line to the last 60% of phase III.7,8,12 Likewise, regression lines were fitted to the points around the inflexion point of phase II within 20% of the time or volume of phase II, to determine their slopes in the mainstream (SII,T,MS and SII,V,MS) and sidestream (SII,T,SS and SII,V,SS) measurements. The angles formed by the phase II and III limbs of the expiratory time mainstream (αMS) and sidestream (αSS) capnograms were calculated from the phase II and phase III slopes using a monitoring speed of 1.67 kPa s−1 (12.5 mm Hg s−1).
Additionally, dead space fractions were calculated from volumetric capnograms. Fowler's dead space, reflecting the volume of the conducting airways,27 was determined by taking the volume expired up to the inflexion point of phase II from the mainstream and sidestream capnograms (VDF,MS and VDF,SS). The physiological dead space according to Bohr (VDB,MS and VDB,SS), reflecting the alveolar volume with decreased or no perfusion, was calculated from the mainstream and sidestream capnograms as follows:28
where and are the mean alveolar partial pressures of CO2 determined from the midpoint of phase III in the mainstream and sidestream capnograms, respectively,3,14 and and are the mixed expired CO2 partial pressure obtained by calculating the area under the mainstream and sidestream volumetric capnogram curves, respectively, via integration and dividing the resulting values by VT.
Enghoff's approach contains all of the ventilation–perfusion mismatching. Hence, besides the VDB, it also incorporates the intrapulmonary shunt (i.e. the alveolar volume with decreased or even loss of ventilation with perfusion maintained), as follows:29
where is the partial pressure of CO2 in the arterial blood.
Additionally, we calculated the normalized differences between the Enghoff and Bohr dead spaces obtained by mainstream [Vs,MS/VT=(VDE,MS−VDB,MS)/VT] and sidestream capnography [Vs,SS/VT=(VDE,SS–VDB,SS)/VT], which reflects the all the mixed venous blood entering the arterial system, including Thebesian veins, part of the bronchial veins, and intrapulmonary shunt circulation (i.e. the virtual gas volume of the alveolar units with perfusion with decreased or no ventilation).
The amount of the CO2 exhaled during each expiration was calculated as the area under the volumetric CO2 concentration curve obtained by mainstream () and sidestream () capnography.
The changes in the sampling flow rate during the mechanical ventilation were measured in a smaller cohort of ventilated patients (n=5). The sampling flow was assessed by measuring the pressure difference between the proximal and distal ends of the sampling tube with a miniature differential pressure transducer (model 33NA002D; ICSensors, Milpitas, CA, USA). The potential variability of the sampling flow governed by the respiratory impedance can theoretically bias the accuracy of sidestream estimates. Thus, the main cohort of patients was divided into three groups based on their compliance (C) values into lower (C<37 ml cm H2O−1) and higher (C>53 ml cm H2O−1) quartiles and medium interquartile range.
Measurement protocol
Mainstream and sidestream capnographic signals were recorded simultaneously during different stages of cardiac surgery: before sternotomy, 5 min before and after cardiopulmonary bypass, and immediately after sternal closure. Two pairs of 15 s traces were recorded in each stage, producing eight recordings per patient (∼20 pairs of expirations). For the assessment of , arterial blood gas samples were obtained during each measurement condition, and the resistance (R) and compliance (C) values displayed by the ventilator were also registered.
Supplemental measurements
To assess whether sidestream capnography affects the mainstream results via gas suctioning, an additional protocol was performed with a set-up identical to that used in the main study group in a smaller cohort of patients (n=8). A total number of 87 mainstream measurements, each lasting 60 s, were performed with the sidestream sampling flow switched on randomly during either the first or second half of the recordings. Separate analyses of first and second halves allowed pairwise comparisons of mainstream capnogram parameters obtained with and without gas suctioning by the sidestream device.
Statistical analyses
Sample size estimation was based on the aim to determine the 95% limits of agreement with great reliability according to the corresponding recommendation.30 The correlations between the mainstream and sidestream variants of individual variables were analysed with the Pearson test. If the regression lines were close to the line of identity for a corresponding mainstream and sidestream value pair, Bland–Altman analysis was performed to assess the extent of their agreement.31 In the event of normality, Student's paired t-tests were used to assess the statistical significance of the difference between the results of the mainstream and sidestream methods. The effects of compliance on the sidestream and mainstream dead space and shunt parameters were assessed by using one-way anova tests on ranks. A P-value <0.05 was considered significant. The reported values are expressed as the mean (sem) in case of normality, or as the median [first quartile–third quartile] otherwise.
Results
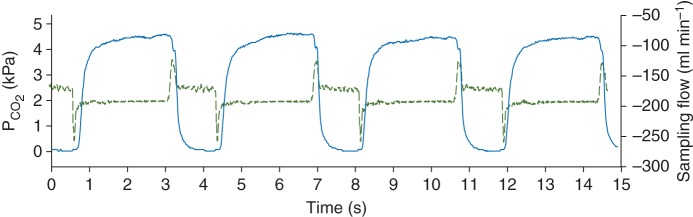
Figure 1 shows representative time and volumetric capnograms obtained with simultaneous mainstream and sidestream capnography. In both the time and volume domains, the mainstream capnograms exhibited a steeper phase II, smaller α angles, and an earlier transition into phase III. Moreover, a later transition into the inspiratory phase was observed in the time domain mainstream capnogram. These shape differences result in a lower area under the sidestream capnogram compared with the corresponding mainstream capnogram.
Fig 1.
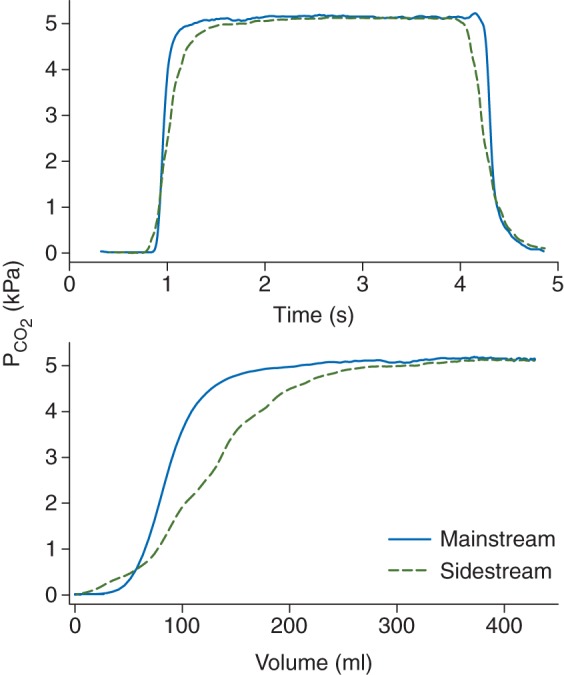
Representative time (top) and volumetric (bottom) mainstream (continuous traces) and sidestream (dashed traces) capnograms.
Figure 2 illustrates the temporal relationship between the sidestream capnogram and the sampling flow variability in a representative patient. The transient spikes in the sampling flow coincide with the cyclic changes in the breathing phases.
Fig 2.
Sidestream capnogram curve (continuous line, left axis) together with the flow in the sampling tube (dashed line, right axis) in a representative patient.
The difference in mainstream and sidestream partial pressure of end-tidal CO2 [; 4.27 (0.02) vs 4.24 (0.02) kPa] was small, although statistically significantly higher with the former technique (P<0.001). The was systematically underestimated by the sidestream method [=0.895 (0.3), P<0.0001], despite the presence of good correlation between these variables (R2=0.91, P<0.0001).
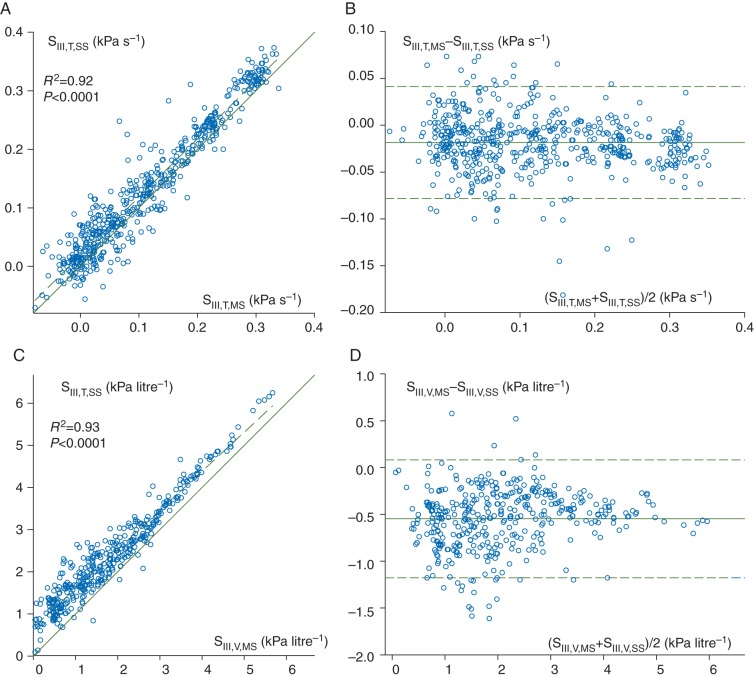
Correlations between the phase III slopes obtained by the mainstream and sidestream methods, and the corresponding Bland–Altman plots, are demonstrated in Fig. 3. An excellent correlation (R2=0.92, P<0.0001) and good agreement were observed between SIII,T,MS and SIII,T,SS, although the sidestream method slightly but significantly overestimated SIII,T [SIII,T,SS/SIII,T,MS=1.05 (0.16), P<0.0001]. Strong correlation and good agreement were found between the volumetric SIII values (R2=0.93, P<0.0001), with a systematic overestimation of SIII,V,MS by SIII,V,SS (SIII,V,SS/SIII,V,MS=1.32 [1.21–1.49], P<0.0001). The limits of agreements were −0.08 to 0.04 kPa s−1 and −0.07 to 1.16 kPa litre−1 for the time and volumetric phase III slopes, respectively.
Fig 3.
Correlations between the phase III slopes in the time (a, SIII,T,MS vs SIII,T,SS) and volumetric domains (c, SIII,V,MS vs SIII,V,SS) obtained by mainstream (horizontal axis) and sidestream capnography (vertical axis), with the regression lines (dashed) and the lines of identity (continuous). Regression equations: SIII,T,SS=0.142+0.996·SIII,T,MS and SIII,V,SS=5.09+0.93·SIII,V,MS. The corresponding Bland–Altman plots are demonstrated on the right for the time (b, SIII,T,MS vs SIII,T,SS) and volumetric (d, SIII,V,MS vs SIII,V,SS) capnograms. The means of differences are −0.019 kPa s−1 and −0.55 kPa litre−1 (continuous), and the limits of agreement are 0.06 kPa s−1 and 0.63 kPa litre−1 (dashed) for the time and volumetric capnograms, respectively. Each data point represents one expiration. SIII,t,ms, phase III slope of time capnogram; SIII,v,ms, phase III slope of volumetric capnogram; SII,t,ms, phase II slope of time capnogram; SII,v,ms, phase II slope of volumetric capnogram; Vdf,ms/Vt, normalized Fowler dead space; Vdb,ms/Vt, normalized Bohr dead space; Vde,ms/Vt, normalized Enghoff dead space; Vs,ms/Vt, normalized difference between the Enghoff and Bohr dead spaces; Vt, tidal volume.
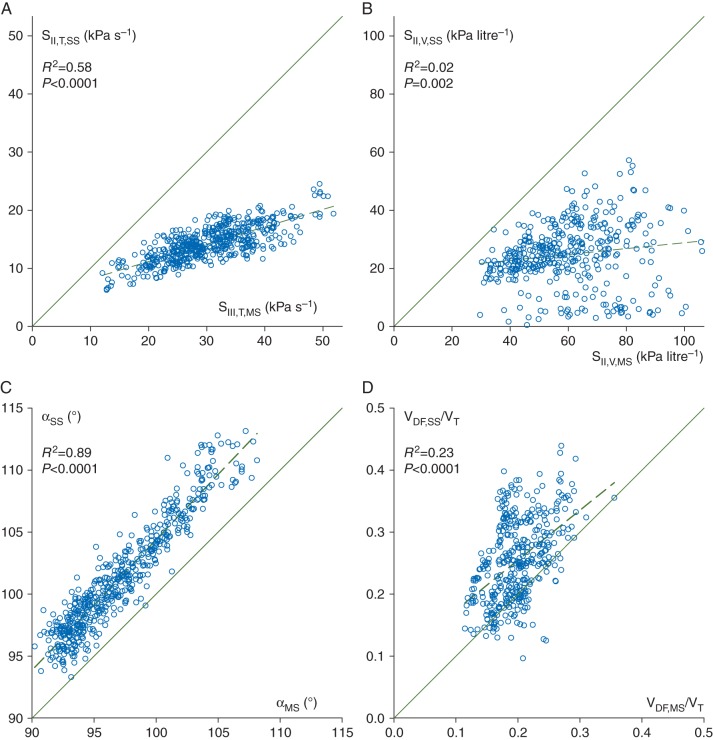
Figure 4 depicts the correlations between the shape factors and the dead space fractions associated with phase II of the capnogram (VDF). Although SII,T,SS correlates significantly with SII,T,MS (R2=0.58, P<0.0001), there is no agreement between these slopes because of the substantial underestimation by the sidestream method [SII,T,SS/SII,T,MS=0.48 (0.004), P<0.0001]. A rather poor correlation and a lack of agreement were observed between the phase II slopes in the volume domain (R2=0.02, P<0.002), with a similar underestimation by sidestream capnography [SII,V,SS/SII,V,MS=0.44 (0.008), P<0.0001]. Significant correlation but poor agreement was found between the two types of α angle (R2=0.89, P<0.0001), with αSS slightly but consistently overestimating αMS [1.04 (0.001), P<0.0001]. Although VDF,MS and VDF,SS correlated moderately (R2=0.56, P<0.0001), their agreement was rather poor, and the sidestream method overestimated the mainstream values [VDF,SS/VDF,MS=1.3 (0.013), P<0.0001].
Fig 4.
Correlations between phase II slopes in the time (a, SII,T,MS vs SII,T,SS) and volume domain (b, SII,V,MS vs SII,V,SS), angles α (c, αMS vs αSS) and Fowler's dead space indices (d, VDF,MS/VT vs VDF,SS/VT) obtained by mainstream (horizontal) and sidestream (vertical) capnography, with the regression lines (dashed) and the lines of identity (continuous). Regression equations: SII,T,SS=39+0.298·SII,T,MS, SII,V,SS=140.2+0.1·SII,V,MS, αSS =−0.84+1.05·αMS, and VDF,SS/VT=−22.3+1.52·VDF,MS/VT. Each data point represents one expiration.
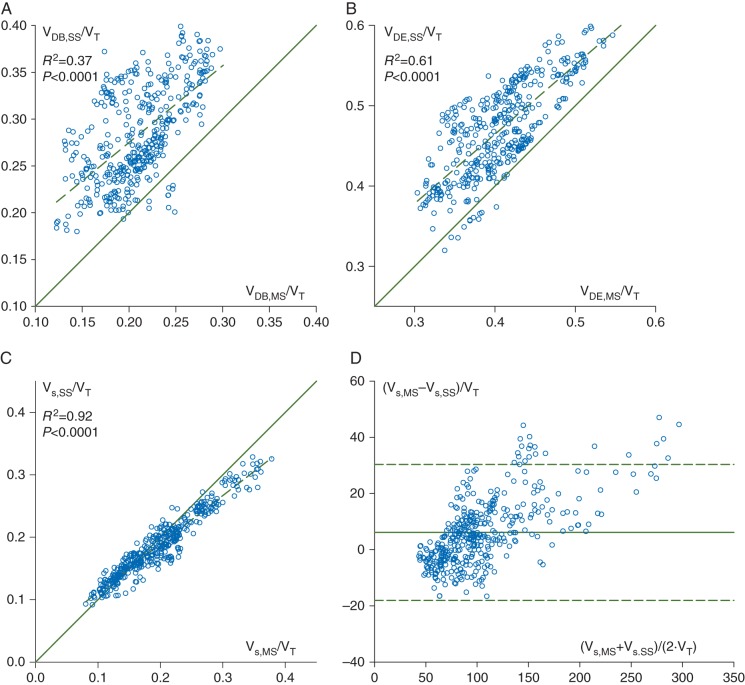
Figure 5 illustrates the correlations between respiratory dead space indices measured by the two methods. Moderate, but statistically significant correlation was found between the normalized dead space parameters VDB,MS/VT and VDB,SS/VT (R2=0.37, P<0.0001), with overestimation of mainstream Bohr's dead space by the sidestream method [VDB,SS/VDB,MS=1.37 (0.01), P<0.0001]. In the measurement of the Enghoff dead space, the two methods exhibited good correlation (R2=0.61, P<0.0001) and a slight overestimation by the sidestream capnograph [VDE,SS/VDE,MS=1.16 (0.004), P<0.0001 ]. Given that shows excellent agreement between the two techniques [R2=0.95 and mainstream/sidestream ratio=1.01 (0.02), P=0.2], the dissociations between physiological dead space parameters can be ascribed to the discrepancies in [R2=0.77 and mainstream/sidestream ratio=1.12 (0.08), P<0.0001]. The overestimations of VDE and VDB by the sidestream technique resulted in a strong correlation in their difference (i.e. the lung volume with the intrapulmonary shunt; R2=0.92, P<0.0001 between Vs,SS/VT and Vs,MS/VT). This relationship was associated with good agreement between the shunt volumes, with the sidestream method only slightly underestimating the mainstream values [Vs,SS/Vs,MS=0.97 (0.004), P<0.0001]. The limit of agreement was 24 ml.
Fig 5.
Correlation between normalized dead space indices calculated according to Bohr (a, VDB,MS/VT vs VDB,SS/VT) and Enghoff (b, VDE,MS/VT vs VDE,SS/VT), and their difference [c, Vs,MS/VT=(VDE,MS–VDB,MS/VT) vs Vs,SS/VT=(VDE,SS–VDB,SS)/VT] obtained by mainstream (horizontal) and sidestream (vertical) capnography, with the regression line (dashed) and the line of identity (continuous). Regression equations: VDB,SS/VT=0.11+0.82·VDB,MS/VT, VDE,SS/VT=0.12+0.86·VDE,MS/VT, and Vs,SS/VT=0.034+0.774·Vs,MS/VT. (d) The corresponding Bland–Altman plot is demonstrated for Vs,MS/VT and Vs,SS/VT. The mean of differences is 6.17 (continuous), and the limit of agreement is 24.2 (dashed). Each data point represents one expiration.
To reveal the effect of lung stiffness on the difference between the dead space and pulmonary shunt parameters determined by the mainstream and sidestream methods, Fig. 6 depicts the differences between mainstream and sidestream values as a function of C. Decreasing compliance resulted in an increasing overestimation of dead space and shunt parameters assessed by using the sidestream technique (P<0.0001).
Fig 6.
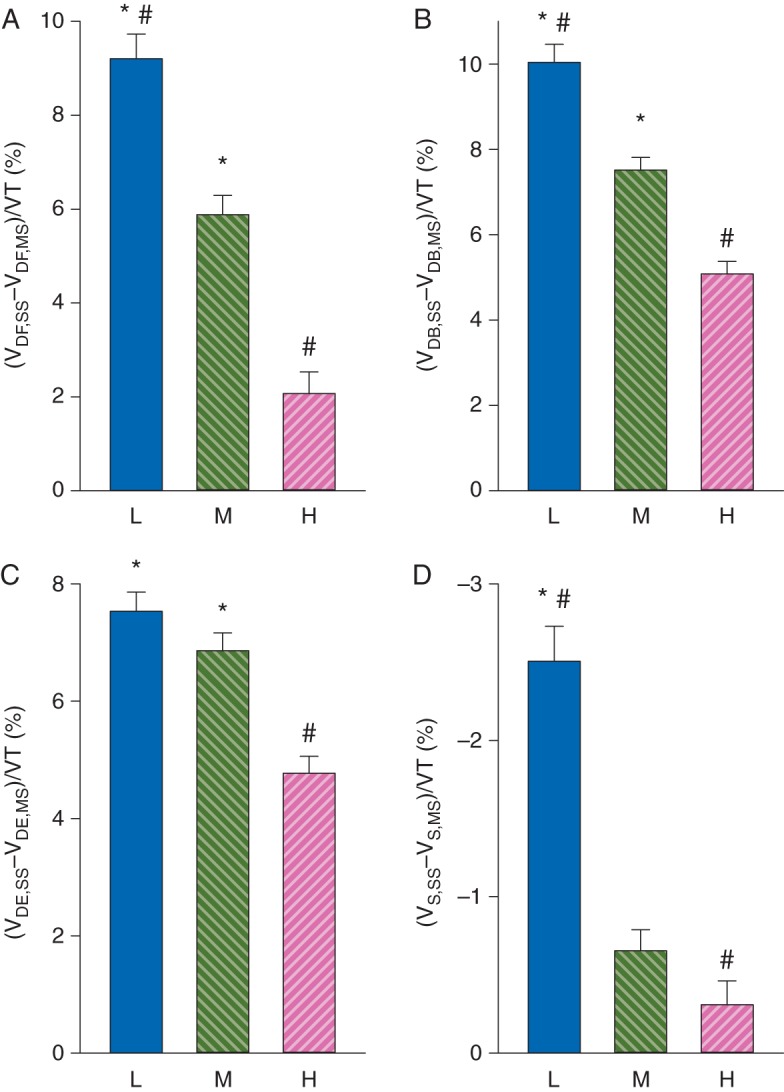
Relative differences in dead space and pulmonary shunt parameters obtained with the two methods in patients with the lower (L, compliance <37 ml cm H2O−1) and higher (H, compliance >53 ml cm H2O−1) quartiles and medium (M) interquartile range. *P<0.05 vs H; #P<0.05 vs M.
In the supplemental measurements assessing the potential biasing effect of sidestream sampling flow on the mainstream parameters, no statistically significant differences were found in any of the mainstream capnogram parameters obtained with or without suctioning (Table 1; P>0.11), whereas a 3.64 (1.22) ml difference was found in VT (P<0.004).
Table 1.
Mainstream capnographic parameter values, obtained with or without sidestream suctioning, and the corresponding P-values of Student's paired t-tests. Values are expressed as the mean (sd). SIII,T,MS, phase III slope of time capnogram; SIII,V,MS, phase III slope of volumetric capnogram; SII,T,MS, phase II slope of time capnogram; SII,V,MS, phase II slope of volumetric capnogram; VDF,MS/VT, normalized Fowler dead space; VDB,MS/VT, normalized Bohr dead space; VDE,MS/VT, normalized Enghoff dead space; Vs,MS/VT, normalized difference between the Enghoff and Bohr dead spaces; VT, tidal volume
| Sidestream suctioning | SIII,T,MS (kPa s−1) | SIII,V,MS (kPa litre−1) | SII,T,MS (kPa s−1) | SII,V,MS (kPa litre−1) | VDF,MS/VT | VDB,MS/VT | VDE,MS/VT | Vs,MS/VT | VT (ml) |
|---|---|---|---|---|---|---|---|---|---|
| On | 0.106 (0.108) | 1.82 (0.92) | 29.13 (5.42) | 62.96 (14.31) | 0.196 (0.037) | 0.198 (0.04) | 0.401 (0.059) | 0.198 (0.067) | 586 (116) |
| Off | 0.109 (0.104) | 1.85 (0.92) | 29.03 (5.36) | 62.19 (14.23) | 0.195 (0.036) | 0.198 (0.04) | 0.403 (0.058) | 0.199 (0.065) | 590 (116) |
| P-value | 0.4 | 0.19 | 0.39 | 0.18 | 0.21 | 0.11 | 0.13 | 0.4 | 0.003 |
Discussion
The results of the present study revealed that the sidestream capnography led to a dynamic distortion of the CO2 concentration curve compared with the mainstream approach regarded as a reference technique.32 Thus, the sidestream method biased the solid indices obtained from capnogram regions in which rapid changes in CO2 concentration occur (i.e. phase II slopes, the transition from phase II to III, the end-tidal portion, , and derived parameters, such as Fowler's and Bohr's dead space). However, the sidestream technique does provide a good approximation of capnogram parameters characterizing periods of low rates of change in CO2 (phase III slopes) and intrapulmonary shunt.
The differences between the sidestream and mainstream techniques can be explained by physical principles. The transport delay of the gas in the sampling tube is a well-described characteristic of the sidestream measurement system.17 This phenomenon introduces a predictable time lag in the detection of the CO2 concentration and gives rise to axial mixing of the gas residing in the sampling tube.2,10,11,19 Axial in-line diffusion in both space and time occurs during the transport, depending on the CO2 gradient.32 This blurring process equilibrates the concentration differences between the gas compartments.26 Theoretically, the biasing effects of this adverse process can be diminished by shortening the sampling tube, increasing the suction flow rate, or both. Shortening the sampling tube (from 3 to 1.5 m) in five additional patients led to fairly proportional improvements in the sidestream estimates to the ratio of the tube lengths (SII,T,SS/SII,T,MS of 37.4 and 60.2%; VDF,SS/VDF,MS of 154.2 and 128.5% with long and short tubes, respectively). Likewise, increasing the sampling flow rate decreased the difference between sidestream and mainstream estimates (SII,T,SS/SII,T,MS of 47 and 76%; SIII,T,SS/SIII,T,MS of 146 and 99% for suction rates of 100 and 350 ml min−1, respectively). These results suggest the possibility of improving the accuracy of shape factor estimates by using sidestream capnography.
A further factor contributing to the distortion of the sidestream capnogram is the variable sampling flow rate resulting from the alternating positive airway pressure during mechanical ventilation.19,20 Given that this phenomenon acts during inspiratory–expiratory phase transitions, it ultimately modifies the ascending and descending limbs of the capnograms.19,20
The physical principles described above are of less importance in the assessment of the capnogram phase III slope. The reason for the good correlation and agreement (Fig. 3a and b) is the relatively steady-state CO2 concentration (Fig. 1) and constant gas sampling flow during this period (Fig. 2). In the only previous study where the sidestream and mainstream phase III slopes were compared, substantially greater differences were observed in infants, which can be attributed to the higher ventilation rate (∼32 bpm).26
The initial part of the capnogram, comprising the phase II slopes, angle α, and VDF, coincides with a high rate of change in the CO2 concentration and with sudden pressure alterations in the breathing circuit causing variable sampling flow rate20 in the tube of the sidestream capnograph. Consequently, in agreement with previous results on ventilated infants,26 the phase II slope of the sidestream capnogram is lower than that obtained by the mainstream technique (Fig. 1, bottom panel, and Fig. 4b). This reduction in SII,T,SS of necessity infers weak relationships between the anatomical dead spaces, VDF,MS and VDF,SS (Fig. 4d), and the sidestream-derived αSS (Fig. 4c).
The ventilation–perfusion mismatch can be divided into alveolar dead space ventilation and shunt perfusion.3,14 We obtained fairly weak correlations and agreements of both the normalized Bohr and Enghoff dead space fractions. The correlation analyses revealed that these dissociations can be ascribed to the discrepancies in the , resulting from the dynamic distortion of the sidestream capnogram (e.g. Fig. 1). Taking the difference between the Enghoff and Bohr dead spaces eliminates these discrepancies, which explains the excellent correlations and good agreement between Vs,MS and Vs,SS (Fig. 5c and d). The differences between the two estimates in the dead space and shunt parameters depend on the level of C, with the greatest deviations in patients with low compliance (Fig. 6). Around the ventilation frequency, the respiratory system impedance is dominated by the elastic forces. Given that low compliance involves higher airway pressures, variations in sampling flow rate are expected to be augmented within the respiratory cycle in the presence of increased stiffness. This implies that the use of dead space parameters determined by the sidestream technique might result in false interpretations. Conversely, the assessment of the shunt fraction is feasible by using sidestream capnography, although a slight underestimation is expected in patients with a less compliant respiratory system.
Our measurements demonstrate that the most frequently used capnogram parameter, the , is underestimated by a value with clinically minimal relevance (0.025 kPa). This concordance between the two techniques supports the conclusions of previous studies.23,25
As a methodological aspect, we assessed whether gas sampling to the sidestream capnograph affects the shape of the mainstream capnogram resulting from the juxtaposed position of the mainstream sensor. However, the lack of differences in any of the mainstream parameters revealed that this effect has negligible impact on the mainstream parameters. This lack of sensitivity can also be anticipated from the amount of aspirated volume being about two orders of magnitude smaller than the VT.
In conclusion, we provide evidence that sidestream capnography allows reliable measurement of , time and volumetric phase III slopes, and the intrapulmonary shunt fraction. Thus, sidestream capnography is suitable for quantification of the unevenness of the alveolar ventilation and the ventilation–perfusion mismatch. However, reliable assessments of the phase II slope, the anatomical and physiological dead spaces, and the rate of elimination of CO2 necessitate the combined application of mainstream volumetric capnography and sophisticated bedside information technology tools.
Authors' contributions
Study design: F.P., B.B.
Patient recruitment: Z.C., B.B.
Custom-made software design for data collection: G.H.F., J.T.
Data collection: A.L.B., Z.C.
Data analysis: A.L.B., F.P., G.H.F., B.B.
Interpretation of data: A.L.B., G.H.F., J.T., B.B.
Writing up the paper: A.L.B., F.P., B.B.
All authors contributed to revising the manuscript critically for important intellectual content.
Funding
Hungarian Scientific Research Grant (OTKA K81179 and K115253); European Union and the State of Hungary; European Social Fund (in the framework of TÁMOP 4.2.6 ‘National Excellence Program’ and TÁMOP-4.2.2.D-15/1/KONV-2015-0024).
Acknowledgements
The authors wish to thank Edit Vigh and Kitti Nevery for contributing to the patient recruitment and data collection.
References
- 1.Ortega R, Connor C, Kim S, Djang R, Patel K. Monitoring ventilation with capnography. N Engl J Med 2012; 367: e27. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JE, Jaffe MB. Capnographic waveforms in the mechanically ventilated patient. Respir Care 2005; 50: 100–8; discussion 108–9 [PubMed] [Google Scholar]
- 3.Tusman G, Sipmann FS, Bohm SH. Rationale of dead space measurement by volumetric capnography. Anesth Analg 2012; 114: 866–74 [DOI] [PubMed] [Google Scholar]
- 4.Bhavani-Shankar K, Moseley H, Kumar AY, Delph Y. Capnometry and anaesthesia. Can J Anaesth 1992; 39: 617–32 [DOI] [PubMed] [Google Scholar]
- 5.Walsh BK, Crotwell DN, Restrepo RD. Capnography/capnometry during mechanical ventilation: 2011. Respir Care 2011; 56: 503–9 [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anesthesiologists. Standards for basic anesthetic monitoring. 2015. Available from http://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf (accessed 14 March 2016)
- 7.Krauss B, Deykin A, Lam A et al. . Capnogram shape in obstructive lung disease. Anesth Analg 2005; 100: 884–8 [DOI] [PubMed] [Google Scholar]
- 8.Babik B, Csorba Z, Czovek D, Mayr PN, Bogats G, Petak F. Effects of respiratory mechanics on the capnogram phases: importance of dynamic compliance of the respiratory system. Crit Care 2012; 16: R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioan I, Demoulin B, Duvivier C et al. . Frequency dependence of capnography in anesthetized rabbits. Respir Physiol Neurobiol 2014; 190: 14–9 [DOI] [PubMed] [Google Scholar]
- 10.Strömberg NO, Gustafsson PM. Ventilation inhomogeneity assessed by nitrogen washout and ventilation-perfusion mismatch by capnography in stable and induced airway obstruction. Pediatr Pulmonol 2000; 29: 94–102 [DOI] [PubMed] [Google Scholar]
- 11.Anderson CT, Breen PH. Carbon dioxide kinetics and capnography during critical care. Crit Care 2000; 4: 207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanch L, Lucangelo U, Lopez-Aguilar J, Fernandez R, Romero PV. Volumetric capnography in patients with acute lung injury: effects of positive end-expiratory pressure. Eur Respir J 1999; 13: 1048–54 [DOI] [PubMed] [Google Scholar]
- 13.Tusman G, Suarez-Sipmann F, Bohm SH et al. . Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med 2006; 32: 1863–71 [DOI] [PubMed] [Google Scholar]
- 14.Tusman G, Sipmann FS, Borges JB, Hedenstierna G, Bohm SH. Validation of Bohr dead space measured by volumetric capnography. Intensive Care Med 2011; 37: 870–4 [DOI] [PubMed] [Google Scholar]
- 15.Tusman G, Bohm SH, Sipmann FS, Maisch S. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 2004; 98: 1604–9 [DOI] [PubMed] [Google Scholar]
- 16.Brunner JX, Westenskow DR. How the rise time of carbon dioxide analysers influences the accuracy of carbon dioxide measurements. Br J Anaesth 1988; 61: 628–38 [DOI] [PubMed] [Google Scholar]
- 17.Breen PH, Mazumdar B, Skinner SC. Capnometer transport delay: measurement and clinical implications. Anesth Analg 1994; 78: 584–6 [DOI] [PubMed] [Google Scholar]
- 18.Schena J, Thompson J, Crone RK. Mechanical influences on the capnogram. Crit Care Med 1984; 12: 672–4 [DOI] [PubMed] [Google Scholar]
- 19.Epstein RA, Reznik AM, Epstein MA. Determinants of distortions in CO2 catheter sampling systems: a mathematical model. Respir Physiol 1980; 41: 127–36 [DOI] [PubMed] [Google Scholar]
- 20.Farmery AD, Hahn CE. A method of reconstruction of clinical gas-analyzer signals corrupted by positive-pressure ventilation. J Appl Physiol 2001; 90: 1282–90 [DOI] [PubMed] [Google Scholar]
- 21.Jaffe MB. Mainstream or sidestream capnography? Respironics White Paper. 2002 Available from http://www.oem.respironics.com/Downloads/Main%20vs%20Side.pdf. (accessed 14 March 2016) [Google Scholar]
- 22.Teixeira Neto FJ, Carregaro AB, Mannarino R, Cruz ML, Luna SP. Comparison of a sidestream capnograph and a mainstream capnograph in mechanically ventilated dogs. J Am Vet Med Assoc 2002; 221: 1582–5 [DOI] [PubMed] [Google Scholar]
- 23.McEvedy BA, McLeod ME, Kirpalani H, Volgyesi GA, Lerman J. End-tidal carbon dioxide measurements in critically ill neonates: a comparison of side-stream and mainstream capnometers. Can J Anaesth 1990; 37: 322–6 [DOI] [PubMed] [Google Scholar]
- 24.Pekdemir M, Cinar O, Yilmaz S, Yaka E, Yuksel M. Disparity between mainstream and sidestream end-tidal carbon dioxide values and arterial carbon dioxide levels. Respir Care 2013; 58: 1152–6 [DOI] [PubMed] [Google Scholar]
- 25.Sakata DJ, Matsubara I, Gopalakrishnan NA et al. . Flow-through versus sidestream capnometry for detection of end tidal carbon dioxide in the sedated patient. J Clin Monit Comput 2009; 23: 115–22 [DOI] [PubMed] [Google Scholar]
- 26.Pascucci RC, Schena JA, Thompson JE. Comparison of a sidestream and mainstream capnometer in infants. Crit Care Med 1989; 17: 560–2 [DOI] [PubMed] [Google Scholar]
- 27.Fowler W. The respiratory dead space. Am J Physiol 1948; 54: 405–16 [DOI] [PubMed] [Google Scholar]
- 28.Bohr C. Über die Lungenatmung. Skan Arch Physiol 1891; 53: 236–8 [Google Scholar]
- 29.Enghoff H. Volumen inefficax: Bemerkungen zur Frage des schädlichen Raumes. Upsala Läkareforen Forhandl 1938; 44: 191–218 [Google Scholar]
- 30.Bland M. 2004 How can I decide the sample size for a study of agreement between two methods of measurement? Available from https://www-users.york.ac.uk/~mb55/meas/sizemeth.htm. (accessed 14 March 2016) [Google Scholar]
- 31.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10 [PubMed] [Google Scholar]
- 32.Gravenstein JS, Jaffe MB, Gravenstein N, Paulus DA. Technical Perpectives. Capnography. Cambridge: Cambridge University Press, 2011; 381–96 [Google Scholar]