Abstract
Setting: Twenty-two districts of Nepal, where intensified case-finding (ICF) activities for tuberculosis (TB) were implemented among risk groups under the TB REACH initiative in collaboration with the National TB Programme from July 2013 to November 2015.
Objectives: To assess the yield of TB screening using an algorithm with smear microscopy followed by Xpert® MTB/RIF.
Design: A descriptive study using routinely collected data.
Results: Of 145 679 individuals screened, 28 574 (19.6%) had presumptive TB; 1239 (4.3%) of these were diagnosed with TB and 1195 (96%) were initiated on anti-tuberculosis treatment. The yield of screening was highest among people living with the human immunodeficiency virus (PLHIV) (6.1%), followed by household contacts (3.5%) and urban slum dwellers (0.5%). Among other risk groups, such as prisoners, factory workers, refugees and individuals with diabetes, the yield was less than 0.5%. The number needed to screen to diagnose an active TB case was 17 for PLHIV, 29 for household contacts and 197 for urban slum dwellers. Of 11 525 patients from ICF and the routine programme, 112 (1%) were diagnosed with multidrug-resistant TB.
Conclusion: There was a substantial yield of TB cases among risk groups such as PLHIV and household contacts. Although the yield in urban slum dwellers was found to be moderate, some intervention should nonetheless be targeted because of the large population and poor access to care in this group.
Keywords: operational research, TB REACH, systematic screening, number needed to screen
Abstract
Contexte : Vingt-deux districts du Népal où des activités intensifiées de recherche des cas (ICF) de la tuberculose (TB) ont été mises en œuvre au sein de groupes à risque sous l'égide du projet TB REACH en collaboration avec le programme national TB entre juillet 2013 et novembre 2015.
Objectifs : Evaluer le rendement du dépistage de la TB grâce à un algorithme basé sur la microscopie de frottis suivie d'un test Xpert® MTB/RIF.
Schéma : Etude descriptive basée sur des données recueillies en routine.
Résultats : Sur un total de 145 679 individus dépistés, 28 574 (19,6%) ont été présumés atteints de TB ; 1239 (4,3%) d'entre eux ont eu une confirmation du diagnostic de TB ; parmi ces derniers, 1195 (96%) ont mis en route un traitement anti-tuberculose. Le rendement a été le plus élevé parmi les personnes vivant avec le virus l'immunodéficience humaine (PVVIH) (6,1%) suivies par les contacts domiciliaires (3,5%) et les habitants des bidonvilles (0,5%). Dans d'autres groupes à risque comme les prisonniers, les travailleurs d'usine, les réfugiés et les diabétiques, le rendement a été inférieur à 0,5%. Le nombre de personnes à dépister (NNS) pour diagnostiquer un cas de TB active a été de 17 pour les PVVIH, de 29 pour les contacts domiciliaires et de 197 pour les habitants des bidonvilles urbains. Sur 11 525 patients émanant soit du programme ICF soit du dépistage de routine, 112 (1%) ont eu un diagnostic de TB multirésistante.
Conclusion : Le rendement en termes de cas de TB dépistés parmi les groupes à risque comme les PVVIH et les contacts domiciliaires a été substantiel. Même si ce rendement a été modeste parmi les habitants des bidonvilles, ceux-ci justifient néanmoins une intervention en raison de leur nombre élevé et de leur médiocre accès aux soins.
Abstract
Marco de referencia: Veintidós distritos de Nepal, en los cuales se ejecutaron actividades de búsqueda intensiva de casos (ICF) de tuberculosis (TB) en los grupos de riesgo, en el marco del proyecto TB REACH en colaboración con el programa nacional contra la TB de julio del 2013 a noviembre del 2015.
Objetivos: Evaluar el rendimiento de la detección sistemática de la TB aplicando un algoritmo que comporta la baciloscopia, seguida de la prueba Xpert® MTB/RIF.
Método: Fue este un estudio descriptivo a partir de los datos recogidos de manera sistemática.
Resultados: De las 145 679 personas en quienes se practicó la detección, en 28 574 hubo una presunción diagnóstica de TB (19,6%); en 1239 de estos pacientes se estableció el diagnóstico de TB (4,3%); e iniciaron el tratamiento 1195 pacientes (96%). El rendimiento diagnóstico fue más alto en las personas viviendo con el virus de la inmunodeficiencia humana (PVVIH, 6,1%), seguidas de los contactos domiciliarios (3,5%) y los residentes en tugurios (0,5%). En otros grupos de riesgo de contraer la TB como los reclusos, los obreros de fábricas, los refugiados o los pacientes diabéticos el rendimiento diagnóstico fue inferior a 0,5%. El número de personas que se debieron examinar con el fin de detectar un caso de TB activa fue 17 en las PVVIH, 29 en los contactos domiciliarios y 197 en los habitantes de los tugurios. De las 11 525 personas examinadas en la ICF y el programa corriente, se diagnosticó TB multiresistente en 112 casos (1%).
Conclusión: La detección sistemática de casos de TB exhibió un alto rendimiento en los grupos de riesgo como las PVVIH y los contactos domiciliarios. Aunque el desempeño en los tugurios urbanos fue moderado, es importante dirigir intervenciones a estos entornos, dado el tamaño de estas poblaciones y su acceso deficiente a la atención de salud.
Tuberculosis (TB) remains a global public health problem with an estimated 9.6 million new cases in 2014, of which only 6 million (63%) were reported.1 This means that 3.6 million new cases (37%) worldwide went undiagnosed or were not reported. The World Health Organization (WHO) End TB strategy for 2016–2030 emphasises early diagnosis, including universal drug susceptibility testing (DST) and systematic screening of contacts and high-risk groups to reduce the case detection gap.2 The WHO also recommends intensified case finding (ICF) among selected risk groups such as household contacts, people living with the human immunodeficiency virus (PLHIV), prisons and other penitentiary institutions and geographically defined sub-populations with high levels of undetected TB, such as urban slum dwellers.3
ICF has been used extensively in a variety of settings, and has been effective in reducing the prevalence, incidence and mortality of TB.4 With the increased use of the rapid Xpert® MTB/RIF test (Cepheid, Sunnyvale, CA, USA) in low- and middle-income countries and its improved sensitivity in detecting TB, inclusion of Xpert in the screening algorithm has demonstrated improvements in case detection.5 Low TB case detection among poor and marginalised groups is one of the primary strategic challenges faced by the Nepal National Tuberculosis Programme (NTP). The NTP identifies slum dwellers, PLHIV, prisoners, refugees and congregate settings such as factories and hostels as high-risk groups.6 In 2014, of 59 000 estimated prevalent cases, only 37 025 cases were notified to the NTP, with a case notification rate (CNR) of 136 per 100 000 population. There was no substantial improvement in the overall CNR for all forms of TB as well as pulmonary bacteriologically confirmed cases (PBC) during 2010–2013.7
Under the TB REACH project, the non-governmental organisation Health Research and Social Development Forum (HERD, Kathmandu, Nepal), in collaboration with the NTP, has been implementing ICF activities focusing on identified high-risk groups using Xpert and light-emitting diode (LED) microscopy fitted in mobile vans since July 2013.
This study aimed to assess the yield of ICF strategies among different risk groups and their impact on the CNR. The specific objectives were 1) to determine the number and proportion of adults screened, diagnosed and initiated on anti-tuberculosis treatment disaggregated by risk group; 2) to determine the number and proportion diagnosed with multidrug-resistant TB (MDR-TB); and 3) to assess the difference in the CNR in the intervention districts before and after the implementation of ICF strategies compared to the difference in the CNR in control districts where there was no ICF.
METHODS
Study design
This is a descriptive study using routinely collected data under the HERD TB REACH project and the Nepal NTP.
General setting
Nepal is a South Asian country of 147 181 km2 with a population of 26494 405.8 The National Tuberculosis Centre (NTC) is the focal point of the NTP, with the District Public/Health Office implementing NTP activities through district hospitals, primary health care centres (PHCCs), health posts and urban health clinics, which are the basic management units (BMUs). As of 2014, there were 4221 health institutions offering DOTS-based services and 581 microscopy centres offering sputum smear microscopy services. Culture and DST services are provided at two sites in the capital city, Kathmandu.7
Study sites
The study sites included 29 municipalities in 22 districts where ICF strategies were implemented and three control districts with no ICF strategy implementation. The intervention and control districts were selected in consultation with the NTC based on high proportions of target groups.
Study population
The study population included urban slum dwellers, factory workers, prisoners, refugees, monks/nuns, PLHIV, household contacts of TB patients and patients with diabetes. All patients with TB diagnosed through routine NTP activities were used to assess the difference in CNR between the intervention and control districts.
Intensified case-finding strategies under the TB REACH programme
Two mobile vans equipped with Xpert and LED microscopy were used in mobile camps for the diagnosis of TB and MDR-TB. Each mobile van was staffed by a laboratory assistant, a driver and a support staff member, who were supported by outreach workers (ORWs) for screening and sputum collection. The mobile van covered one district every 3 months for 5–7 days. Based on the volume of risk groups, however, the van was mobilised for a maximum of 15 days per district. Prior to the mobilisation of the van, community mapping was conducted by the ORWs to identify high-risk groups in each intervention site.
In addition to the mobile clinics, two static hospital-based sites using Xpert provided services for presumptive TB patients identified in regular out-patient departments (OPD) in addition to the targeted population. During the course of implementation, referrals were also sent to the static sites from central hospitals and the private sector. The different risk groups and the strategies used to reach them are shown in Table 1. Any individual among the risk groups with a cough of ⩾2 weeks was considered a presumptive TB patient and evaluated (see algorithm in the Figure) initially by sputum smear microscopy, followed by Xpert for those who tested smear-negative. In the case of household contacts and PLHIV, a cough of any duration was considered to be presumptive TB. Those cases diagnosed with TB were referred to the nearest BMU for treatment under the NTP.
TABLE 1.
Intensified TB case-finding strategies for various risk groups under the HERD TB REACH project in 22 districts of Nepal, July 2013–November 2015
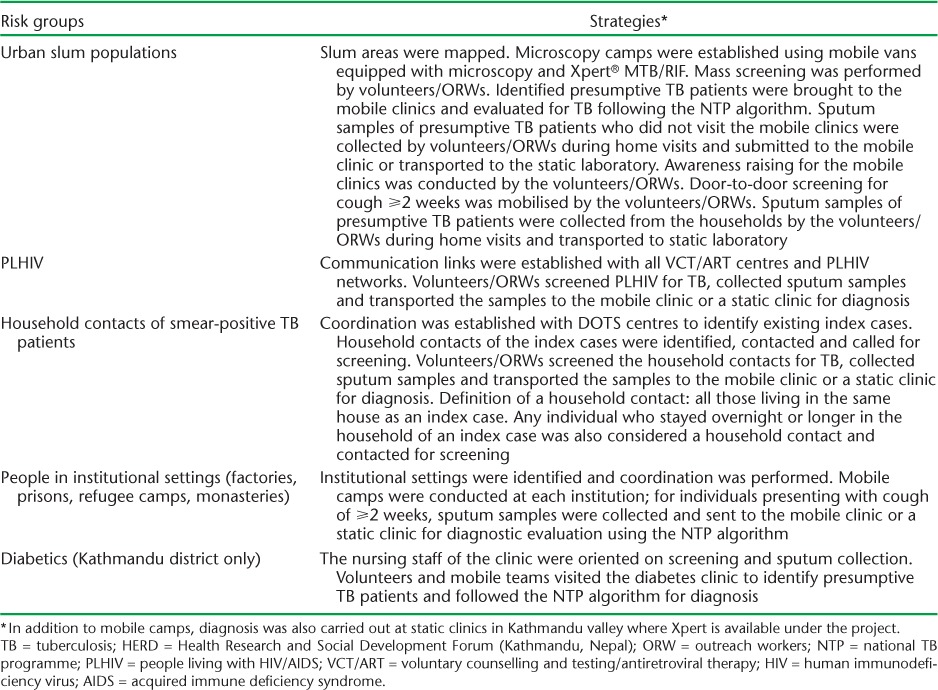
FIGURE.
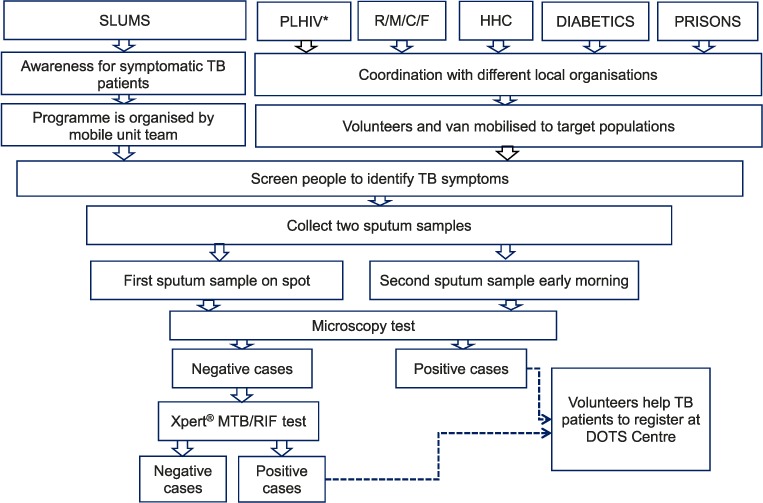
Algorithm of intensified case-finding strategies for TB used for different risk groups for screening and testing in Nepal, 2013–2015. * For PLHIV, sputum samples were tested simultaneously with smear microscopy and Xpert. PLHIV = people living with the human immunodeficiency virus; R/M/C/F = refugees/monasteries/convents/factory workers; HHC = household contacts; TB = tuberculosis.
Data variables and data sources
For each risk group, aggregate numbers of the people screened, those with presumptive TB, those diagnosed with TB and those initiated on anti-tuberculosis treatment were extracted from the quarterly reports of the TB REACH project. The aggregate number of persons diagnosed with MDR-TB was collected from the Xpert register. The CNRs (all forms and PBC) for the intervention and control districts were extracted from the NTP annual report for the 12 months before the intervention (July 2012–June 2013) and during the implementation period (July 2014–June 2015).
Data entry and analysis
The data were entered and analysed using Microsoft Excel (Microsoft Corp, Redmond, WA, USA). The frequencies and proportions of the persons screened, diagnosed and initiated on anti-tuberculosis treatment were calculated from the data. The yield of the ICF was calculated by the total number of diagnosed TB cases in each risk group divided by the total risk population screened. The number needed to screen (NNS) to diagnose a TB case was calculated by taking the inverse of the prevalence of active TB in that risk group. The differences in the changes in the CNR between the two time periods (2012–2013 and 2014–2015) in the intervention and control districts were compared.
Ethics
The study protocol was reviewed and approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. Permission to use the data was obtained from the Nepal NTP, Kathmandu, Nepal.
RESULTS
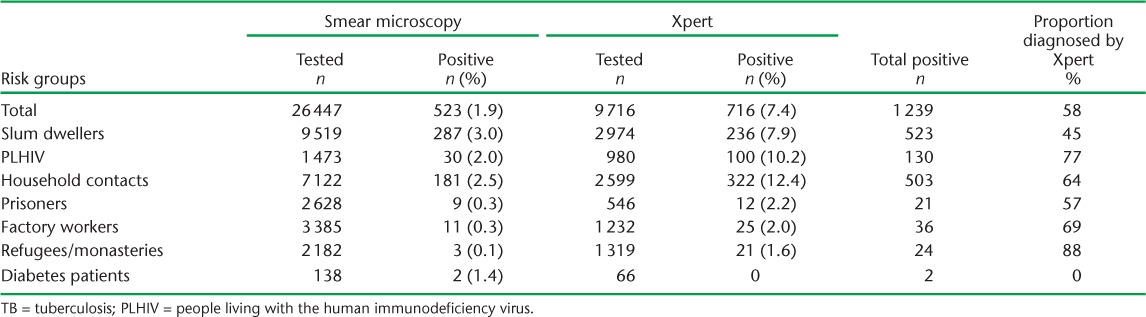
Of 145 679 individuals from different risk groups who were screened for TB in the intervention districts, 28 574 (19.6%) had presumptive TB. Of these, 26 447 underwent sputum microscopy. Of the 25 924 individuals with sputum-negative samples, only 9716 (37%) were tested on Xpert, of whom 716 (7%) tested positive. Of 1239 individuals diagnosed with TB on Xpert and sputum microscopy, 96% were enrolled for anti-tuberculosis treatment; some of these were from outside the intervention district. An additional 2468 individuals were referred from central hospitals or the private sector for Xpert testing, of whom 404 tested positive, resulting in a total of 1643 positive cases. The distribution of TB diagnosis using smear microscopy and subsequent Xpert across the different risk groups is shown in Table 2. In addition to the 9716 presumptive TB patients identified during ICF, 1809 patients were referred for Xpert due to presumed drug-resistant TB. Of 11 525 patients, 112 (1%) were diagnosed with MDR-TB. The sputum samples of 16 208 individuals (63%) were not tested for TB on Xpert.
TABLE 2.
NNS for the groups tested by SM alone and by SM and Xpert® MTB/RIF for those with smear-negative results among risk groups in 22 districts of Nepal, 2013–2015
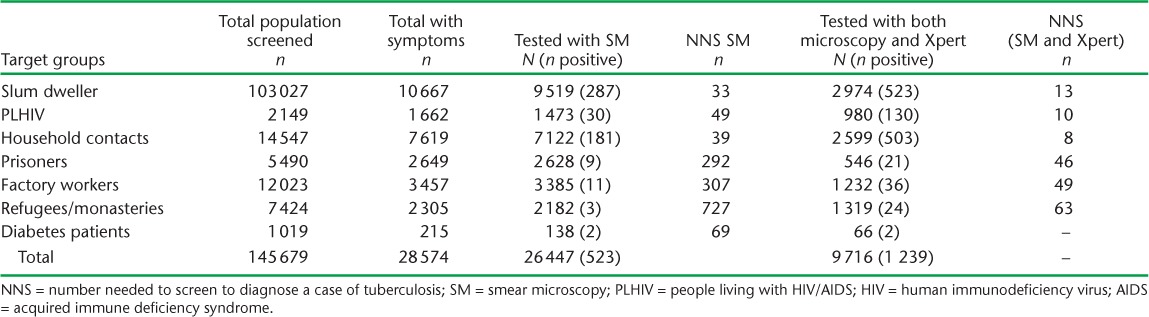
The yield was highest among PLHIV (6.1%), followed by household contacts (3.5%), and was 0.5% or less among the other risk groups. Hence, the NNS to diagnose a case of TB was 17 for PLHIV, 29 for household contacts and 197 for urban slum dwellers (Table 3). Overall, of the total diagnosed TB cases, 58% were diagnosed on Xpert. This proportion was higher in PLHIV (77%) and household contacts (64%) (Table 4).
TABLE 3.
Yield and number needed to screen under intensified case-finding activities for TB among risk groups in 22 districts of Nepal, 2013–2015
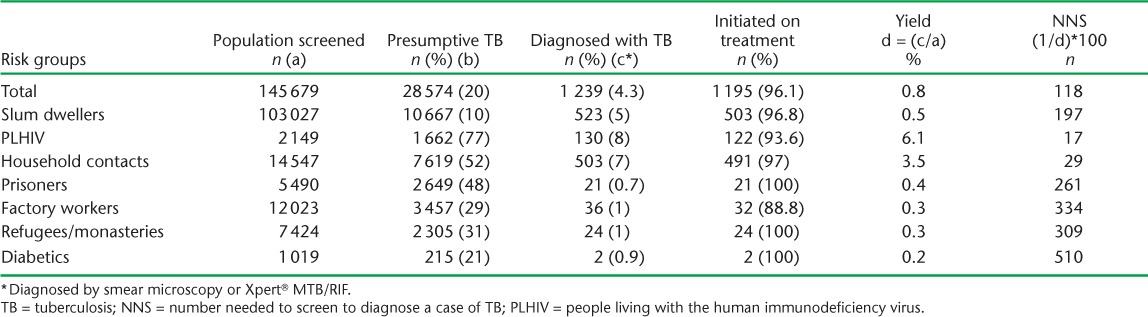
TABLE 4.
Diagnosis of TB under intensified case-finding activities stratified by smear microscopy and Xpert® MTB/RIF in 22 districts of Nepal, 2013–2015
The CNR in the intervention districts for new PBC and all forms of TB before the intervention was respectively 65 and 160 vs. 64 and 145 after the intervention. This indicates a decrease in the CNR by 1/100 000 for new PBC and 15/100 000 for all forms of TB between the baseline and endline periods.
In the control districts, the baseline CNR was 59 for new PBC and 154 for all forms of TB compared to respectively 58 and 149 for the endline CNR, with a decrease of 1/100 000 for new PBC and of 5/100 000 for all forms of TB during the two periods.
DISCUSSION
The study showed a higher yield from screening among PLHIV, household contacts and urban slum dwellers. More than half of the TB cases under ICF were diagnosed on Xpert, and would have gone undiagnosed if Xpert had not been used. If all individuals with smear-negative results had undergone Xpert testing, the yield could have been higher.
The study has the following strengths: it is the first study from Nepal to employ innovative approaches with a mobile van equipped with Xpert and LED microscopy to reach previously unreached populations in large geographical areas. It employed community mapping using local ORWs to help identify target groups, thus increasing access to modern TB diagnostic services. In the context of the limited access to modern diagnostic services within the routine NTP, the availability of a mobile van in the study sites encouraged referrals from the private sector, thus reinforcing the NTP strategy of public-private partnerships. Having the intervention embedded in routine NTP settings increased the feasibility for scale-up, which has been planned in the upcoming NTP five-year strategy.
Our study shows a high yield among PLHIV and household contacts, and is comparable to other studies conducted in moderate prevalence countries.8–12 The NNS to diagnose a case of TB was 17 for PLHIV and 29 for household contacts. This is comparable with the NNS reported in a systematic review, which reported an NNS of 13 (2–120) for PLHIV and 25 (3–568) for household contacts.13 In our study, about two thirds of PLHIV and about half of household contacts had presumptive TB, whereas a study from Ethiopia reported a proportion of PLHIV with presumptive TB of 39%.14 The differences in the definition of presumptive TB across studies makes comparison difficult.
In our study, more than half of the TB cases were diagnosed by Xpert using the same sputum samples on which smear microscopy had been undertaken and declared smear-negative. This again confirms the increased sensitivity of Xpert in diagnosing TB compared to conventional microscopy, even in field settings. In practice, however, not all the smear-negative patients were tested on Xpert; the major reasons for this were the higher caseloads on Xpert due to mass screening and the inability to test all the smear-negative cases on Xpert as the maximum number of tests that could be performed per day by the Xpert 4 module was 24, in six lots.
Although ICF activities showed substantial yield among a few risk groups, the CNR for both PBC and all forms of TB decreased in both the intervention and control districts. The decrease in the CNRs is directly related to the decrease in the overall national CNR by 11% in 2014–2015 compared to 2012–2013.15 Furthermore, as the TB REACH Project only covered targeted areas of selected municipalities, some of the cases were enrolled in adjoining districts and were not included in the overall CNR of the intervention district. In addition, the NTP placed an Xpert machine in one of the control districts, and this may have contributed to the lower decrease in the CNR in the control districts. Furthermore, a number of intervention districts were highly affected by the earthquake of April 2015, which brought a temporary halt to the project ICF activities and possibly the routine NTP activities in the intervention districts.
The total yield is driven by both the size of the risk group in the general population and the prevalence of TB within that risk group. The groups with the highest risk for TB, such as PLHIV and household contacts, are often also the smallest, and groups with only a moderately elevated risk such as urban slum dwellers and diabetes patients can be very large. The success of ICF activities thus depends on many factors, including the proportion of undiagnosed cases in the risk groups, the size and coverage of the risk groups and the algorithm used in screening.
Previous studies have shown the usefulness of ICF and its role in reducing TB incidence, prevalence and mortality in different settings.16 The impact of the ICF activities in several districts on improving childhood TB CNR was documented recently in Nepal.17 However, ZAMSTAR, a community-randomised trial in Zambia and the Western Cape province of South Africa, showed that neither community-level enhanced TB case finding nor household level TB-HIV care led to a statistically significant reduction in the TB burden in the community. It is important to note that the trial involved only enhanced case finding, and not systematic screening (active screening), as was done in the present study.18
There were limitations in this study. As only smear-negative cases underwent Xpert testing, some smear-positive results may have been false positives due to the low sensitivity of smear microscopy. We could have reduced the number of false-positives if we had used Xpert as a confirmatory test for the smear-positive patients. Xpert coverage was not optimal, and more than half of the presumptive TB patients who were smear-negative did not undergo testing; this could have underestimated the yield in all the target groups, as the sputum collected by the volunteers was not of optimal quality to be tested on Xpert. There were some differences in the coverage of the Xpert testing, ranging from 21% among prisoners to 68% for PLHIV (Table 4).
In active case finding, false-positive results may be substantial and generate unnecessary costs and exposure to toxic medicines for patients.19 For the smear-positive patients, false positivity may have been an issue that was not assessed in our study, although the microscopy quality control assessed by the NTP shows 100% agreement. As volunteers and ORWs were involved in the identification of individuals with presumptive TB in the risk groups, errors could have occurred when asking about symptoms, leading to a decreased yield. The coverage of each risk group was not assessed, as valid estimates were not available in the district. As a great deal of human and material resources were involved in the ICF activities, a cost-effectiveness study would have helped to assess the usefulness of the interventions.
The study has several policy implications. First, considering the yield among PLHIV and household contacts in a resource-limited setting, the ICF activities using a mobile van equipped with Xpert should be targeted at these groups for better utilisation of resources. Strengthening the existing strategies, including ICF, in these groups would enhance early case detection, reducing disease complications and mortality. Second, although the study showed a moderate yield among urban slums, the findings do not provide sufficient evidence to prove the effectiveness of ICF among slum dwellers. Systematic screening could, however, be initiated, considering the large population size and poor health-seeking behaviour of urban slum dwellers.20 This would help improve case detection, reducing delays in treatment initiation and also improving the early detection and management of drug-resistant TB.
In conclusion, there was a substantial yield of TB cases in the different risk groups through the use of the ICF strategies. Although in the short term there was no increase in the CNR, considering the benefits of early case detection, systematic screening could be implemented for urban slum dwellers. Further studies should look into the best ICF delivery models in both high-risk and non high-risk groups, considering that access to care may limit efforts to control TB.
Acknowledgments
The authors would like to thank all those who supported this project and article. The data were taken from the programme ‘Using Gene Xpert MTB/RIF to reach the unreached: mobile diagnostic case finding in Nepal’ a TB REACH project delivered by the Health Research and Social Development Forum (HERD, Kathmandu, Nepal), in collaboration with the National Tuberculosis Programme (Kathmandu, Nepal). The authors would also like to acknowledge the respective District Health Officers, volunteers and district staff involved in the project for their support throughout the implementation of the programme.
The research for this study was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR), Geneva, Switzerland. The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and Médecins Sans Frontières (MSF), Brussels Operational Centre, Luxembourg. The specific SORT IT programme that resulted in this publication was jointly developed and implemented by The Union South-East Asia Regional Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LUXOR), MSF; the School of Public Health, Post Graduate Institute of Medical Education and Research, Chandigarh, India; and the Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
The implementation of the programme was funded by Global Affairs Canada (Ottawa, ON, Canada) through TB REACH, a global initiative of the Stop TB Partnership (Geneva, Switzerland). The writing programme was funded by the Department for International Development, London, UK. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report, 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. [Google Scholar]
- 2.World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 3.World Health Organization Improving early case detection of active TB through systematic screening. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.04. [Google Scholar]
- 4.World Health Organization. WHO Three I's meeting. Intensified case finding, isoniazid preventive therapy and TB infection control for people living with HIV. Geneva, Switzerland: WHO; 2008. Report of a joint World Health Organization HIV/AIDS and TB Department meeting. April 2–4, 2008. http://www.who.int/hiv/pub/tb/3is_mreport/en/ Accessed May 2016. [Google Scholar]
- 5.Piatek A S, Van Cleeff M, Alexander H et al. GeneXpert for TB diagnosis: planned and purposeful implementation. Glob Health Sci Pract. 2013;1:18–23. doi: 10.9745/GHSP-D-12-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nepal Ministry of Health and Population. National Tuberculosis Programme. Annual Report FY 2069/70 (2012/2013) Thimi, Bhaktapur, Nepal: National Tuberculosis Centre, Ministry of Health and Population, Government of Nepal; 2014. [Google Scholar]
- 7.Nepal Ministry of Health and Population. National Tuberculosis Programme, Annual Report FY 2070/71 (2013/2014) Thimi, Bhaktapur, Nepal: National Tuberculosis Centre, Ministry of Health and Population, Government of Nepal; 2015. [Google Scholar]
- 8.World Health Organization. World Health Statistics 2014. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 9.Nsanzumuhire H, Aluoch J A, Karuga W K et al. A third study of case-finding methods for pulmonary tuberculosis in Kenya, including the use of community leaders. Tubercle. 1981;62:79–94. doi: 10.1016/0041-3879(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 10.Jackson-Sillah D, Hill P C, Fox A et al. Screening for tuberculosis among 2381 household contacts of sputum-smear-positive cases in The Gambia. Trans R Soc Trop Med Hyg. 2007;101:594–601. doi: 10.1016/j.trstmh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Zachariah R, Spielmann M P, Harries A D et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–1039. [PubMed] [Google Scholar]
- 12.Mangi R, Pfau R, Ahmad S et al. Incidence of child tuberculosis among household contacts of all types of TB patients: a 4-year study. Int J Tuberc Lung Dis. 2010;14(Suppl 2):S58. [Abstract FA–101202–13] [Google Scholar]
- 13.Shapiro A, Chakravorty R, Golub J, Akande T, Lönnroth K. A systematic review of the number needed to screen to detect a case of active tuberculosis in different risk groups. Geneva, Switzerland: WHO; 2013. http://www.who.int/tb/Review3NNS_case_active_TB_riskgroups.pdf Accessed May 2016. [Google Scholar]
- 14.Adelman M W, Tsegaye M, Kempker R R et al. Intensified tuberculosis case finding among HIV-infected persons using a WHO symptom screen and Xpert MTB/RIF. Int J Tuberc Lung Dis. 2015;19:1197–1203. doi: 10.5588/ijtld.15.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nepal Ministry of Health and Population. National Tuberculosis Programme Annual Report FY 2071/72 (2014/2015) Thimi, Bhaktapur, Nepal: National Tuberculosis Centre, Ministry of Health and Population, Government of Nepal; 2016. [Google Scholar]
- 16.World Health Organization. Systematic screening for active tuberculosis: an operational guide. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.6. [Google Scholar]
- 17.Joshi B, Chinnakali P, Shrestha A. Impact of intensified case finding strategies on childhood TB case registration in Nepal. Public Health Action. 2015;5:93–98. doi: 10.5588/pha.15.0004. et.al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayles H, Muyoyeta M, Du Toit E et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–1194. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 19.van't Hoog A H, Onozaki I, Lönnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:532. doi: 10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United Nations Human Settlements Programme/World Health Organization. Hidden cities: unmasking and overcoming health inequities in urban settings. Geneva, Switzerland: WHO/UN-HABITAT; 2010. http://www.who.int/kobe_centre/publications/hidden_cities2010/en/ Accessed May 2016. [Google Scholar]


