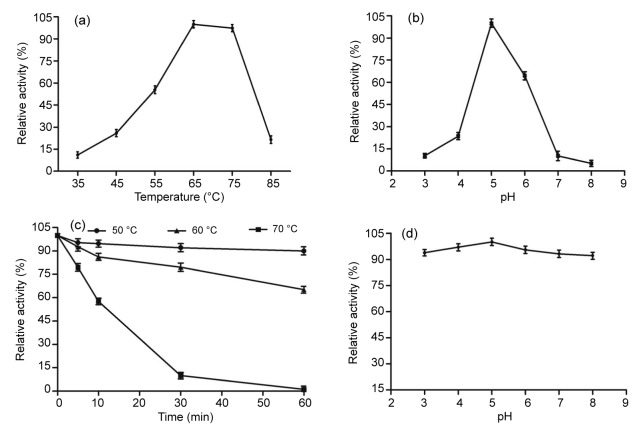
Fig. 2.
Characterization of the purified A. terreus β-glucosidase
(a) Effect of temperature on A. terreus β-glucosidase activities. The activity was measured in McIlvaine buffer (100 mmol/L, pH 5.0) using pNPG as the substrate. (b) Effect of pH on A. terreus β-glucosidase activities. The enzyme activities at various pH values were measured at 50 °C for 10 min using pNPG as the substrate. (c) Thermostability of A. terreus β-glucosidase. The residual activity was measured using pNPG as the substrate after pre-incubation without substrate at 50, 60, and 70 °C for different periods of time. (d) pH stability of A. terreus β-glucosidase. The residual activities were measured at optimal conditions against pNPG after incubation at various pH values, 25 °C for 1 h. The error bars represent the standard deviation (SD), with n=3