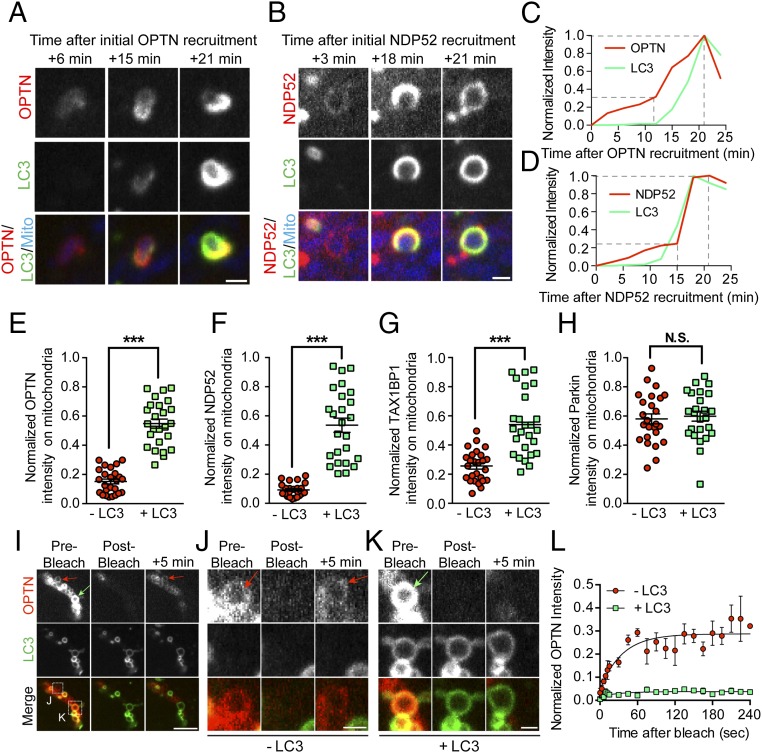
Fig. 4.
Autophagy receptors are recruited and stabilized on damaged mitochondria in a two-step process. (A and C) Before LC3 binding, OPTN is weakly recruited to fragmented mitochondria, gradually increasing in intensity over time. When LC3 is recruited ∼15 min later, OPTN intensity markedly increases before stabilizing with the formed autophagosome at 25 min after initial recruitment. (B and D) As with OPTN, NDP52 is initially weakly recruited to fragmented mitochondria. NDP52 binding to fragmented mitochondria is enhanced as LC3 rings form and stabilize ∼25 min after initial recruitment. Dashed lines indicate steps of autophagy receptor recruitment. (E–G) At 90 min post-CCCP, mitochondrially localized autophagy receptors display significantly higher normalized intensity on LC3-positive mitochondria compared with LC3-negative mitochondria. (H) At this time point, mitochondrially localized Parkin intensity is not affected by autophagosome formation. (I–K) To probe the stability of OPTN on mitochondria with or without LC3, OPTN rings on depolarized mitochondria were bleached with 640-nm laser light. (I) Confocal time series shows OPTN fluorescence recovery on LC3-negative mitochondria (red arrows) but not LC3-positive mitochondria (green arrow) after photobleaching. (J) Magnified view of boxed region in I shows clear recovery of OPTN signal intensity 5 min after 640-nm laser bleaching (red arrows). (K) Magnified view of boxed region in I shows little recovery of OPTN signal 5 min postbleaching (green arrow). (L) LC3-negative mitochondria display significantly higher fluorescence recovery after photobleaching. (Scale bars: I, 5 μm; A, B, J, and K, 1 μm.) In E–H, n = 50 mitochondria from five different cells per comparison. Error bars represent mean ± SEM. In L, fluorescence recovery of OPTN is shown in which three mitochondria per condition were fit to a single exponential association model. ***P < 0.001. N.S., not significant.