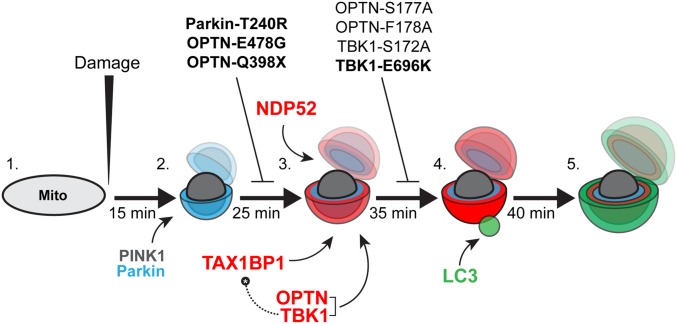
Fig. 10.
Model depicting autophagy receptor recruitment to depolarized mitochondria over time. (1) Before a depolarizing injury, mitochondria are elongated and motile. (2) Within 15 min of injury, damaged mitochondria recruit PINK1 and Parkin, resulting in the phosphoubiquitination of outer membrane proteins and mitochondrial fragmentation. (3) Twenty-five minutes after initial damage, and within ∼10 min of the first Parkin recruitment, autophagy receptors OPTN, TAX1BP1, and NDP52 are weakly recruited to the surface of fragmented mitochondria. OPTN shuttles its upstream kinase TBK1 to the mitochondrial surface. Expression of the Parkinson’s disease-associated Parkin-T240R mutant blocks recruitment of all three receptors. Additionally, ALS-linked OPTN-E478G and OPTN-Q398X mutants are not recruited to ubiquitinated mitochondria. (4) Within 5–10 min of weak autophagy receptor recruitment, a small number of mitochondria display enhanced autophagy receptor recruitment and stabilization, coincident with the recruitment of the autophagosome membrane protein LC3. This step is dependent on TBK1 phosphorylation of OPTN S177, and is blocked in cells expressing ALS-linked TBK1-E696K. Although OPTN and TAX1BP1 depend on TBK1 activity in this pathway, NDP52 can stabilize on mitochondria and facilitate autophagic engulfment of mitochondria even in the absence of TBK1. Thus, high levels of NDP52 can compensate for OPTN or TBK1 loss of function. (5) By 40–45 min after the initial damage, fully formed autophagosomes develop around mitochondria, effectively sequestering the damaged organelles from the surrounding cytosol. Bolded mutants have been linked to neurodegenerative disease.