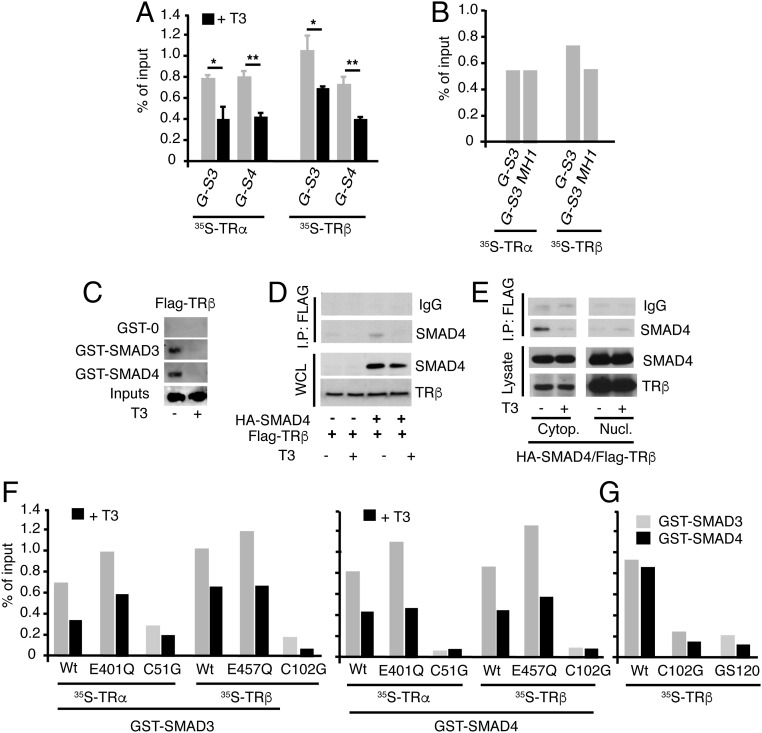
Fig. 3.
Interaction of TRs with SMADs. (A) Quantifications of pull-down assays with GST-SMAD-3 (G-S3) and GST-SMAD4 (G-S4) and in vitro translated TRα or TRβ in the presence and absence of T3. Data are expressed as percentage of the inputs and are mean ± SD from three independent assays. Significant differences in the absence and presence of T3 are indicated. (B) GST pull-downs of TRα and TRβ with SMAD3 or its N-terminal MH1 fragment. Data were quantitated from two independent assays. (C) GST or GST-SMADs were incubated with 700 μg whole extracts of Cos-1 cells transfected with a Flag-tagged TRβ and treated in the presence and absence of 50 nM T3 for 1 h. The bound receptor was analyzed by Western blot. The input represents 10% of the proteins used in the binding assay. (D) HeLa cells were transfected with HA-SMAD4 and Flag-tagged TRβ, and 36 h later, they were treated with 50 nM T3 for 1 h. Immunoprecipitates (700 µg) with 3 µg anti-Flag antiserum or with a control serum (IgG) were subjected to Western analysis, together with 10% of the cell extract (input) with SMAD4 or TRβ antibodies. (E) Cells were transfected with HA-SMAD4 and Flag-tagged TRβ, treated with T3, and cytoplasmic and nuclear fractions were prepared and immunoprecipitations were performed as in D. (F) Quantification of in vitro GST pull-down assays of SMAD3 and SMAD4 with the indicated TRα and TRβ point mutants in the LBD and the DBD. Assays were performed in the presence and absence of T3 (G) Comparison of interaction of the wild-type receptor with the indicated DBD mutants in GST pull-down assays. Results of F and G represent the mean values obtained in two independent assays.