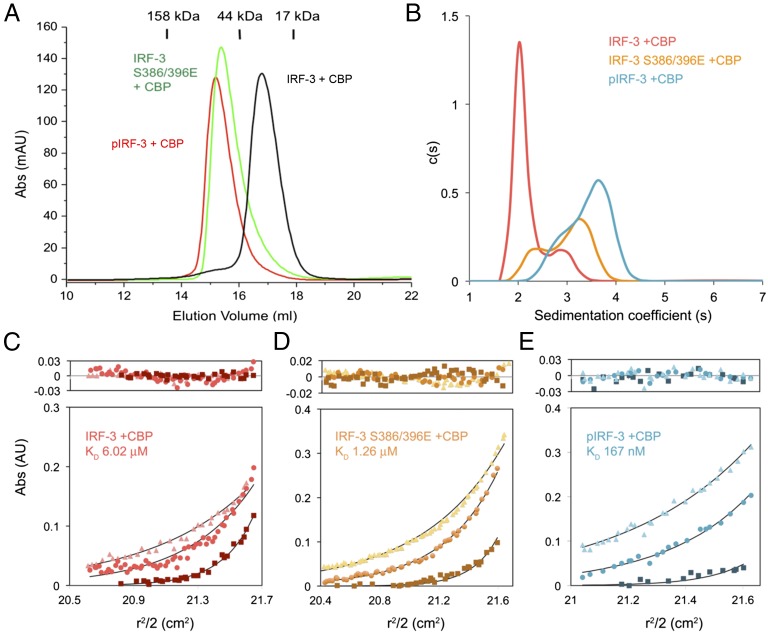
Fig. 3.
The IRF-3 phosphomimetic mutant S386/396E in complex with CBP dimerizes in solution. (A) Gel-filtration chromatography analyses of IRF-3/CBP complexes. The black curve is for unphosphorylated IRF-3 bound to CBP. The green chromatogram is for the IRF-3 S386/396E mutant bound to CBP. The red curve is for the TBK1 pIRF-3/CBP complex. (B) Sedimentation velocity AUC analysis of the samples described in A. The data are consistent with a shift from primarily monomeric IRF-3 in the unphosphorylated state toward the dimeric state upon phosphorylation. (C–E) Sedimentation equilibrium AUC analyses of the three IRF-3/CBP complex samples. Protein samples were spun at 18,000, 22,000, and 32,000 rpm in a Beckman An-60 Ti rotor at loading concentrations of 1, 3.5, and 14 μM. Nine datasets for each sample were analyzed globally; for clarity, three representative data curves for each sample are shown. The data are best described by an equilibrium between 1:1 and 2:2 IRF-3:CBP complexes, with experimental Kd values listed on each plot. In each panel, data from 18,000, 22,000, and 32,000 rpm rotor speeds are shown by squares, circles, and triangles, respectively.