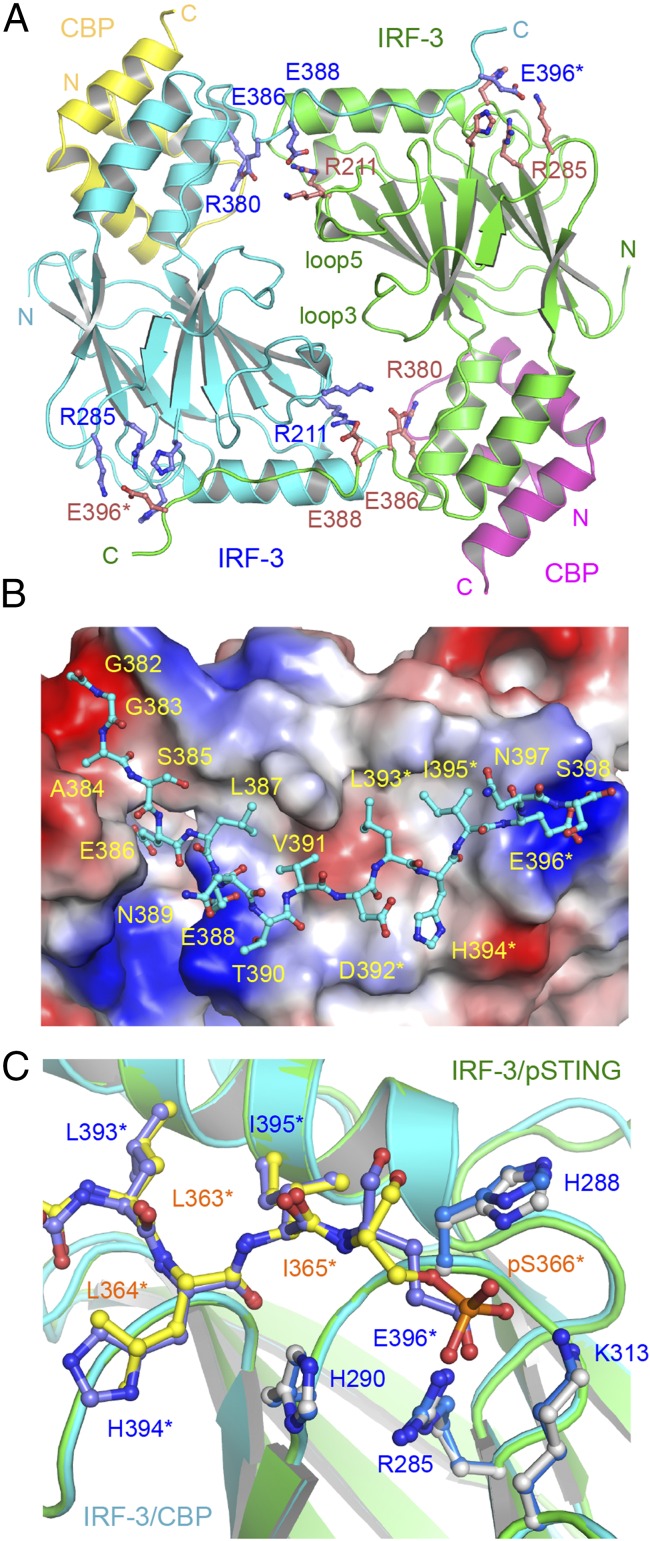
Fig. 4.
Structure of the IRF-3 phosphomimetic mutant S386/396E bound to CBP. (A) The domain-swapped dimer of the IRF-3 S386/396E mutant bound to CBP. Key residues mediating IRF-3 dimerization are shown by blue and pink ball-and-stick models. Residues of the pLxIS motif are indicated by asterisks. (B) Structure of the C-terminal tail of IRF-3 (cyan ball-and-stick model) that mediates IRF-3 dimerization. The IRF-3 dimer with the C-terminal tail omitted is shown by the surface representation colored according to surface electrostatic potential. The positively charged surface is in blue, and the negatively charged surface is in red. (C) Superposition of the pLxIS motifs of IRF-3 and pSTING. The pLxIS motif of IRF-3 with the S396E mutation is shown by the blue ball-and-stick model. The pLxIS motif of pSTING is shown by the yellow ball-and-stick model.