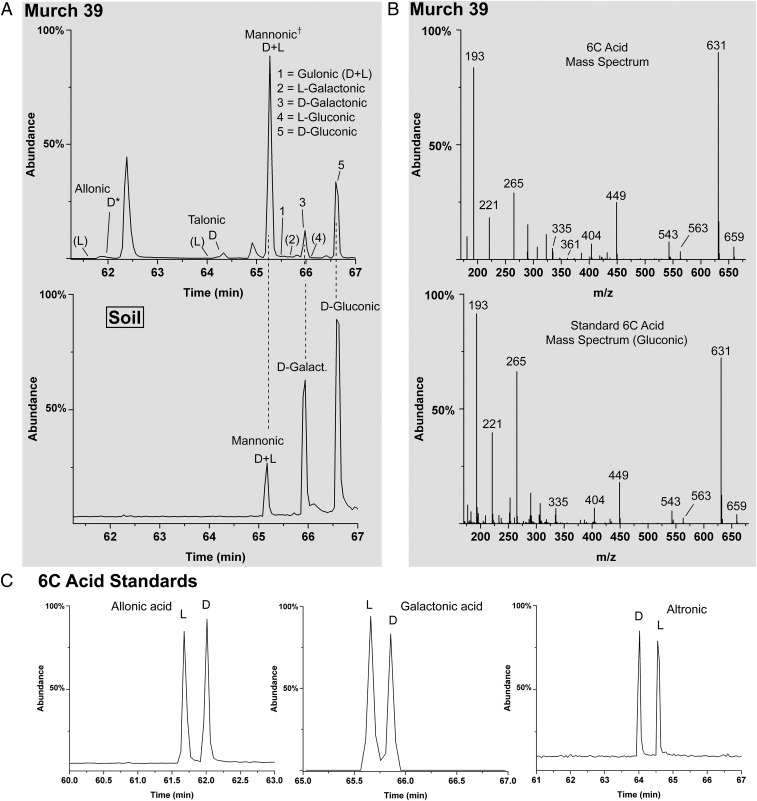
Fig. 5.
GC-MS chromatograms and mass spectra of 6C sugar acids; all compounds in this analysis were converted to their i-Pr/TFA derivatives. (A) Murch 39 (Upper) and the corresponding compounds from soil/dust (Lower, from same soil/dust sample as Figs. 4B and 3E); *, in Murch 39, d-allonic acid is of relatively low abundance and is considered a tentative identification; however, the major ions, 631, 563, and 543, are present in the mass spectrum (see B) at the correct GC retention time; †, the enantiomers of mannonic acid coelute under the employed GC conditions. Parentheses indicate that the l enantiomers were not detected; their known retention times are indicated. (B) Mass spectra of a 6C standard (gluconic acid) (Lower) and a 6C sugar acid (Upper) from Murchison. The shown mass spectra are nearly identical to all 6C aldonic acids. (C) Examples of enantiomer separation of standards.