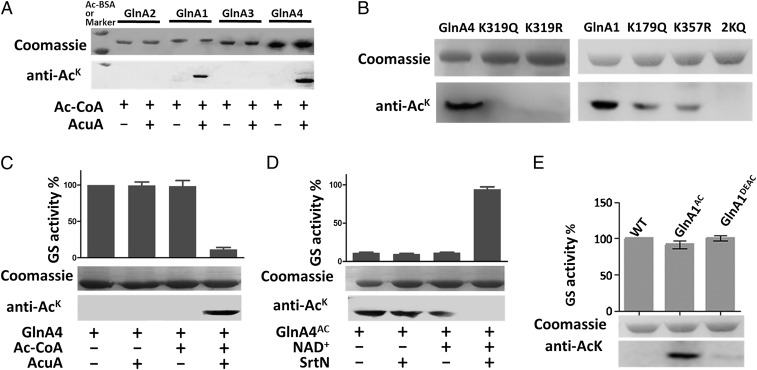
Fig. 2.
Acetyltransferase AcuA acetylates two GSs (GlnA1 and GlnA4) in S. erythraea. (A) The four purified GS enzymes (GlnA2, GlnA1, GlnA3, and GlnA4) were incubated in vitro with or without AcuA and AcCoA at 37 °C for 2 h. After incubation, samples were collected and analyzed by SDS polyacrylamide gel electrophoresis, and the acetylation levels were determined by Western blotting using a specific anti-AcK antibody. (B) GlnA1, GlnA4, and their variants (GlnA1K179Q, GlnA1K357Q, GlnA1K179,357Q, GlnA4K319Q, and GlnA4K319R) were acetylated by AcuA. (C and D) In vitro acetylation/deacetylation affected the activity of GS. GlnA4 was incubated with AcuA and AcCoA at 37 °C for 2 h. Acetylated GlnA4 was incubated with SrtN and NAD+ at 37 °C for 2 h. Error bars show the SD from three independent experiments. (E) GS activity of native GlnA1 (WT), GlnA1AC, and deacetylated GlnA1 (GlnA1DEAC).