Figure 13. Sildenafil enhances the ATPase inhibitory effects of regorafenib, sorafenib, pazopanib, AR-13 and OSU-03012.
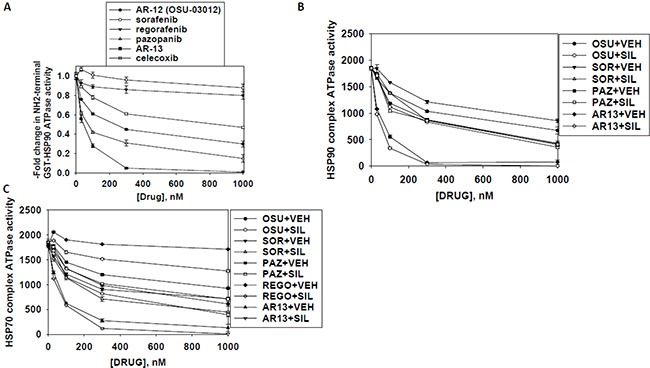
(A) A GST-HSP90 NH2-terminal fragment containing the ATP binding domain of the chaperone was synthesized in E. coli and purified from other bacterial proteins using glutathione sepharose. The GST-HSP90 NH2-terminal fragment protein was not eluted off the sepharose beads. Equal portions of beads were immediately aliquoted into individual wells in a 96 well plate. Beads were resuspended in kinase reaction buffer containing vehicle control; OSU-03012; sorafenib tosylate; regorafenib; pazopanib; AR-13; celecoxib (30 nM; 100 nM; 300 nM; 1 μM) in triplicate, and incubated for 30 min at 37°C. The reaction was started by addition of ATP-lite substrate. The plate was removed from the incubator and placed into a Vector 3 plate reader to determine the luminescence of the reactions under each treatment condition (n = 3 (× 3) +/− SEM). (B and C) GBM12 cells were transfected with a plasmid to express HSP70-GFP or to express FLAG-tagged HSP90. Twenty four h after transfection cells were treated with vehicle control or sildenafil (2 μM) for 1 h. Chaperone proteins were immuno-precipitated using their tags in the presence of phosphatase inhibitors. Equal portions of precipitate sepharose beads were immediately aliquoted into individual wells in a 96 well plate. Beads were resuspended in ATPase reaction buffer containing vehicle control; OSU-03012; sorafenib tosylate; regorafenib; pazopanib; AR-13; (30 nM; 100 nM; 300 nM; 1 μM) in triplicate, and incubated for 30 min at 37°C. The reaction was started by addition of ATP-lite substrate. The plate was removed from the incubator and placed into a Vector 3 plate reader to determine the luminescence of the reactions under each treatment condition (n = 3 (× 3) +/− SEM).
