Figure 7. Analysis of the effects of Rituximab and Trastuzumab on CDC and monocyte/macrophage reactivity.
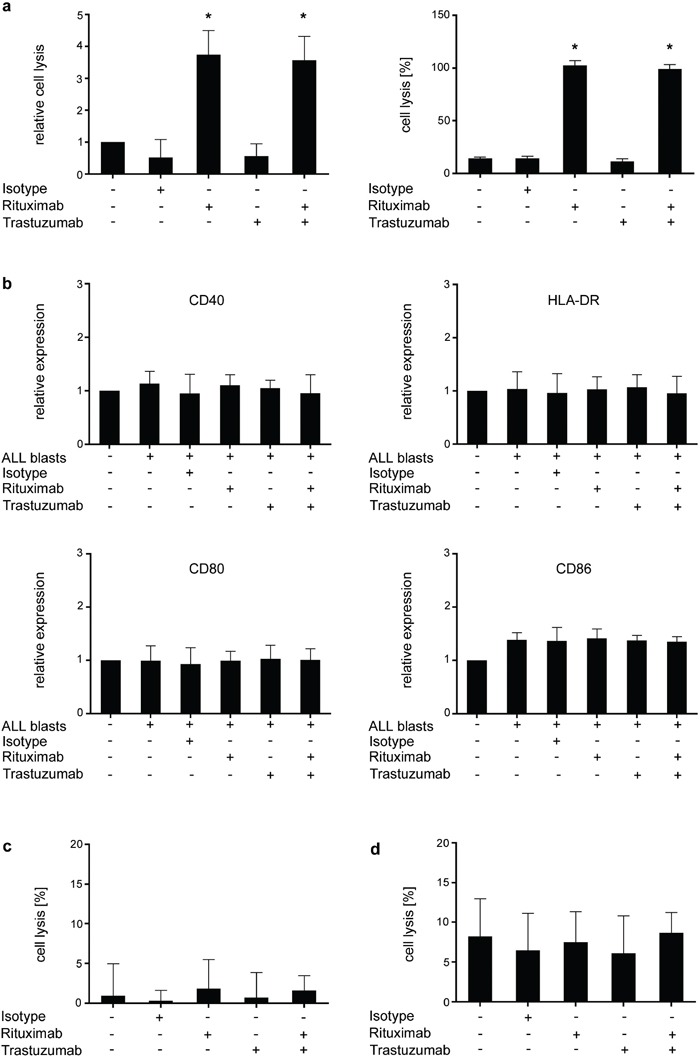
PBMC of ALL patients (CD20+HER2/neu+) were left untreated or incubated with or without 10 μg/ml of Rituximab, Trastuzumab, a combination of both or isotype control in the presence or absence of serum a. monocytes b, c. or macrophages d. (a) After 2 hour incubation, CDC was determined by BATDA Europium assays. Results of single experiments were normalized to the medium control. Relative blast lysis was calculated as multiples of medium control. Results obtained in 4 independent experiments (left panel) and representative results of one exemplary experiment (right panel) are shown. Statistics were calculated in comparison with the medium control. (b) Monocyte activation as reflected by upregulation of CD40, CD80, CD86 and HLA-DR was measured by flow cytometry after 16 hours of incubation with or without antibodies or isotype control. Pooled data of 3 independent experiments are shown. Surface antigen expression (MFI) was normalized to untreated monocytes (set to 1) and calculated as changes from baseline expression. Statistics were calculated in comparison with untreated monocytes. (c) Monocyte-mediated lysis of ALL blasts was determined after 2 hour incubation with or without antibodies or isotype control (10 μg/ml each) by BATDA Europium assays. Pooled data of 3 independent experiments are shown. (d) Macrophage-mediated lysis of ALL blasts was determined after 2 hour incubation with or without antibodies or isotype control (10 μg/ml each) by BATDA Europium assays. Pooled data of 3 independent experiments are shown. Error bars represent means and SD. Statistically significant results are indicated by *.
