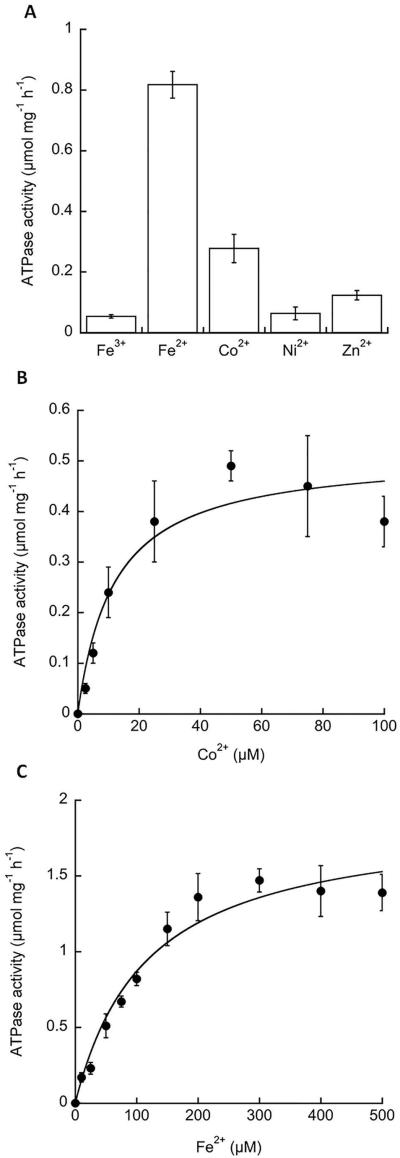
Fig 5. Activation of FrvA ATPase activity by metal ions.
A. FrvA was purified and its ATPase activity (μmol mg−1 h−1) was measured in vitro in the presence of 100 μM of metal ions as indicated.
B. Kinetic characterization of the FrvA ATPase activity in the presence of various concentrations of Co(II) reveals a Vmax = 0.51 ± 0.05 μmol mg−1 h−1 and K1/2 = 12 ± 4 μM.
C. Kinetic characterization of the FrvA ATPase activity in the presence of various concentration of Fe(II) reveals a Vmax = 1.88 ± 0.14 μmol mg−1 h−1 and K1/2 = 116 ± 24 μM.
Data are expressed as the mean ± SD (n=3).