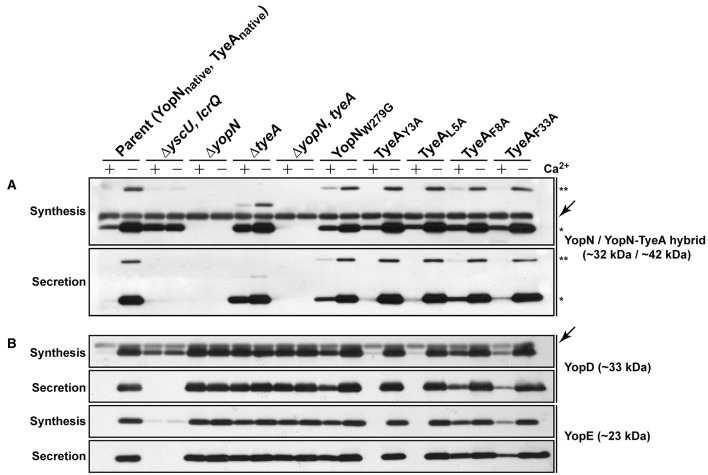
Figure 8.
The YopNW279-TyeAF8 contact is required for controlled Yop synthesis and secretion by in vitro grown Yersinia. Bacteria were grown in BHI medium either with (+) or without (−) Ca2+. Collected samples consisted of a mix of proteins contained within intact bacteria and associated with the outer bacterial surface that were retained in the bacterial pellet (Synthesis) or Yop proteins secreted free into the extracellular medium obtained from the cleared culture supernatants (Secretion). These were fractionated on a long 12% SDS-PAGE, wet-blotted onto PDVF membrane and then analyzed by immunoblot using polyclonal rabbit anti-YopN antiserum (A) or polyclonal rabbit anti-YopD and anti-YopE antiserum (B). The single asterisk (*) highlights the singular YopN (~32 kDa) polypeptide, while the double asterisk (**) reveals the naturally produced and secreted ~42 kDa YopN-TyeA hybrid. Arrows (←) indicate non-specific protein bands recognized by the anti-YopN antiserum and the anti-YopD antiserum. The band appearing just above the nonspecific band in the ΔtyeA strain likely represents a frameshifting event that causes full-length YopN to be fused with the TyeAΔ19−59 deletion remnant resulting in a hybrid product that has a predicted molecular weight of ~38 kDa. Strains: Parent (YopNnative, TyeAnative), YPIII/pIB102; ΔyscU, lcrQ double mutant, YPIII/pIB75-26; ΔyopN null mutant, YPIII/pIB82; ΔtyeA null mutant, YPIII/pIB801a; ΔyopN, tyeA double mutant, YPIII/pIB8201a; YopNW279G, YPIII/pIB8223; TyeAY3A, YPIII/pIB8221; TyeAL5A, YPIII/pIB8222; TyeAF8A, YPIII/pIB8220; TyeAF33A, YPIII/pIB8219. The theoretical molecular masses predicted from amino acid sequence are given in parentheses.