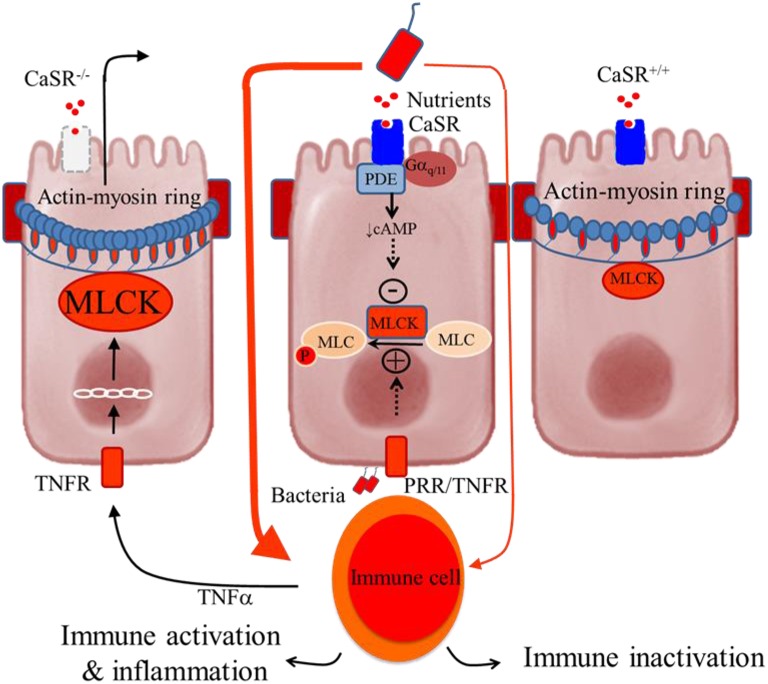
Figure 2.
Schematic representation of the colonocytes showing how deficiency in intestinal CaSR results in increased gut permeability and inflammation. Central panel, illustrates a current model of self-amplifying pathway for intestinal disease (Turner, 2006) where a small amount of luminal bacteria or bacteria-derived molecules pass across the epithelium to activate lamina propria immune cells, leading to secretion of proinflammatory cytokines (e.g., TNFα) and subsequent activation of their receptors (e.g., TNFR) in the epithelium. The latter increases MLCK transcription and activity and phosphorylation of myosin light chain (MLC), resulting in increased contractility of perijunctional actin-myosin ring and increased epithelial permeability. The consequences are greater access leakage of luminal materials, greater immune activation, and even greater barrier defects. The presence of CaSR ligands and signals limits amplifying of this cascade through activation of phosphodiesterase (PDE) (Geibel et al., 2006) and inhibition of MLCK (Cheng et al., 2014), leading to MLC dephosphorylation and barrier stabilization. Thus, in the presence of intact CaSR signaling, as in CaSR+∕+ mice, immune tolerance or only low-grade inflammation is seen (Right panel). However, in the absence of CaSR signal, as in CaSR−∕− mice, the limiting of this cascade amplification is lost, leading to immune activation and uncontrolled inflammation (Macleod, 2013b; Cheng et al., 2014) (Left panel).