Abstract
Pyrazinamide (PZA) is a prodrug requiring conversion to pyrazinoic acid (POA) by an amidase encoded by pncA for in vitro activity. Mutation of pncA is the most common cause of PZA resistance in clinical isolates. To determine whether the systemic delivery of POA or host-mediated conversion of PZA to POA could circumvent such resistance, we evaluated the efficacy of orally administered and host-derived POA in vivo. Dose-ranging plasma and intrapulmonary POA pharmacokinetics and the efficacy of oral POA or PZA treatment against PZA-susceptible tuberculosis were determined in BALB/c and C3HeB/FeJ mice. The activity of host-derived POA was assessed in rabbits infected with a pncA-null mutant and treated with PZA. Median plasma POA values for the area under the concentration-time curve from 0 h to infinity (AUC0–∞) were 139 to 222 μg·h/ml and 178 to 287 μg·h/ml after doses of PZA and POA of 150 mg/kg of body weight, respectively, in mice. Epithelial lining fluid POA concentrations in infected mice were comparable after POA and PZA administration. In chronically infected BALB/c mice, PZA at 150 mg/kg reduced lung CFU counts by >2 log10 after 4 weeks. POA was effective only at 450 mg/kg, which reduced lung CFU counts by ∼0.7 log10. POA had no demonstrable bactericidal activity in C3HeB/FeJ mice, nor did PZA administered to rabbits infected with a PZA-resistant mutant. Oral POA administration and host-mediated conversion of PZA to POA producing plasma POA exposures comparable to PZA administration was significantly less effective than PZA. These results suggest that the intrabacillary delivery of POA and that producing higher POA concentrations at the site of infection will be more effective strategies for maximizing POA efficacy.
INTRODUCTION
The control of tuberculosis (TB) is jeopardized by the increasing prevalence of multidrug-resistant TB (MDR-TB) caused by strains which are resistant to at least rifampin (RIF) and isoniazid (INH) (1). Unfortunately, resistance to the other key sterilizing drug, pyrazinamide (PZA), is commonly present in MDR-TB isolates (2). Compounding this problem are the well-recognized challenges in performing PZA susceptibility testing, resulting in the common therapeutic conundrum of whether to include PZA in MDR-TB treatment regimens without certainty of its potential contribution (3). Whereas the mechanism of action of PZA remains partially unknown, the primary mechanism of PZA resistance in clinical isolates is well established (4, 5). Because PZA is a prodrug that requires conversion to pyrazinoic acid (POA) for its antimicrobial effect, mutations of the gene encoding the mycobacterial PncA amidase (pncA) result in PZA resistance (4–7). Approximately 78% of PZA-resistant isolates harbor mutations in pncA (3). However, since pncA mutations do not affect susceptibility to POA in vitro, direct administration of POA or delivery in a form that does not require PncA for the generation of POA inside Mycobacterium tuberculosis could circumvent this mechanism of resistance.
The MIC of POA against M. tuberculosis is 16 to 32 μg/ml in 7H10 media at pH 5.8 (8) and between 62 and 496 μg/ml at pH 6.5 in 7H9 or 7H11, which may be up to 4-fold lower than the PZA MIC (9). Past studies suggested that POA was not orally bioavailable or was rapidly metabolized to inactive products (10). This steered subsequent investigators toward development of new POA derivatives with greater oral bioavailability and intrapulmonary delivery of POA. However, it is possible that the drug must be metabolized by the bug and concentrated locally and therefore may not be effective even after successful systemic administration. Therefore, it remains important to distinguish the reasons for oral POA failure in past experiments and whether it can be effective if administered systemically. Recent data indicate that POA is, in fact, orally bioavailable. The same study showed that host-mediated conversion of PZA to POA occurs and that POA easily diffuses in tissues (9), reviving interest in POA as a therapeutic solution for MDR-TB patients.
To explore whether POA delivered systemically or derived from host-mediated conversion of PZA could produce in vivo activity comparable to that of PZA, we determined the pharmacokinetics (PK) of POA in the plasma, epithelial lining fluid (ELF), and lung lesions of C3HeB/FeJ mice after oral administration of PZA and POA and compared the activity of PZA and POA after oral administration in different mouse models of TB. To further test whether POA generated from PZA by the host could reduce lung bacillary burdens, we infected rabbits with a pncA-null mutant and treated them with PZA, which in rabbits leads to the accumulation of POA in plasma and tissues at levels higher than PZA (9).
(Portions of the results of this study have been presented previously at the International Workshop on the Clinical Pharmacology of Tuberculosis Drugs, San Diego, CA, September 2015.)
MATERIALS AND METHODS
Mycobacterial strains.
M. tuberculosis H37Rv was used as a frozen stock prepared from a log-phase culture in Middlebrook 7H9 broth after mouse passage and was diluted in 7H9 broth supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase; Becton Dickinson, Franklin Lakes, NJ) before infection. To isolate a PZA-resistant pncA mutant of M. tuberculosis, M. tuberculosis strain HN878 was grown in 7H9 broth containing 0.05% (wt/vol) Tween 80 (Sigma-Aldrich) and 10% oleic acid-albumin-dextrose-catalase (OADC) to an optical density at 580 nm of 1.3 to 1.4. Approximately 1011 bacterial cells were plated on Middlebrook 7H10 agar (Becton Dickinson) containing PZA (Sigma-Aldrich) at a final concentration of 400 μg/ml and supplemented with 0.5% (vol/vol) glycerol (Sigma-Aldrich) and 10% OADC. The plates were then incubated for 4 to 6 weeks. The resulting individual colonies were cultured in 7H9 broth and streaked onto 7H10 agar before the genomic DNA was extracted, as described previously (11). The genotype of the pncA mutant was determined by direct Sanger sequencing using a BigDye terminator sequencing kit (Applied Biosystems), and the reactions were analyzed with an ABI 3100 genetic analyzer. One HN878 mutant with a deletion from position −11 to position +2, resulting in a frameshift of the coding region of pncA, was retained for subsequent infection experiments (HN878PZA400-C1). The MICs of POA were similar against the wild-type HN878 strain and the pncA mutant, as expected.
Drugs and chemotherapy.
PZA was obtained from Acros Organics (Thermo Fisher Scientific, Branchburg, NJ) and formulated for oral administration in distilled water for mice. POA was obtained from Sigma-Aldrich (St. Louis, MO) and formulated for oral administration in distilled water or 40% sucrose. The highest concentrations were warmed at 70°C before administration to ensure dissolution (stability of POA at this temperature was verified by liquid chromatography-tandem mass spectrometry). PZA and POA were administered to mice by oral gavage once (QD) or twice (BID) daily, 5 days per week. PZA was formulated for rabbits in 40% sucrose and administered by oral gavage once daily, 7 days a week, at 175 mg/kg of body weight.
Mouse and rabbit aerosol infection with M. tuberculosis.
All mouse procedures were approved by the Animal Care and Use Committee of Johns Hopkins University. Female BALB/c mice (Charles River, Wilmington, MA) and C3HeB/FeJ mice (Jackson, Bar Harbor, ME) were aerosol infected using an Inhalation Exposure System (Glas-Col, Terre Haute, IN) with dilution of a log-phase culture of M. tuberculosis (optical density at 600 nm of 0.5) to implant approximately 1,000 to 3,000 CFU in the lung for the subacute infection model in BALB/c mice and with dilutions of a titered frozen stock to implant approximately 100 to 300 CFU for the chronic infection model in BALB/c mice and 50 CFU for the chronic infection model in C3HeB/FeJ mice. One day (D1) after infection, two mice from each aerosol run were humanely killed to determine the number of bacteria implanted.
Pathogen-free female New Zealand White rabbits were purchased from Covance (Princeton, NJ). All rabbit procedures were performed according to protocols approved by the Rutgers Institutional Animal Care and Use Committee. Thirty-six rabbits were infected with a titered frozen stock of the pncA mutant HN878PZA400-C1 using an aerosol nose-only delivery system to implant ∼1,000 CFU in the lung. At 3 h after exposure, four rabbits were sacrificed to enumerate the number of bacterial CFU implanted in the lungs at the time of infection.
PK studies. (i) Administration of PZA.
Two pharmacokinetic (PK) studies were performed in C3HeB/FeJ mice. Plasma and ELF concentration-time profiles of POA were determined after a single dose of PZA in uninfected mice and after multiple doses in infected mice. PZA and POA concentrations were assayed in samples of plasma and ELF from all mice and from lung lesions of infected mice obtained, depending on the study, at 0.08, 0.25, 0.45, 1.5, 3, 5, 7, 12, and 18 h after PZA dosing. Three to six mice from each dose group were sampled at each time point. Plasma was obtained either by tail vein bleeding or by cardiac puncture after anesthesia by isoflurane inhalation. ELF was obtained after centrifugation of bronchoalveolar lavage (BAL) fluid, as previously described (12). Lung lesions were obtained by resecting a segment of the lung containing one or two tubercles, depending on their size, and dissecting away as much normal-appearing lung tissue as necessary to obtain at least 20 mg of lesion material. Lungs were rinsed in cold phosphate-buffered saline (PBS) first and then dissected on dry ice to prevent POA diffusion.
(ii) Administration of POA.
Dose-ranging PK studies were performed in both infected and uninfected C3HeB/FeJ and BALB/c mice after single doses of POA. POA concentrations were assayed in plasma samples obtained 0.25, 1.5, 3, 5, 7, and 11 h after dosing, and ELF samples were obtained 3, 5, 7, and 11 h after dosing. All samples were harvested and frozen at −80°C before being shipped to the Dartois laboratory, Rutgers New Jersey Medical School, for quantification.
(iii) Quantification of POA in biological fluid samples.
Quantification of POA was performed as previously described using POA standards from Acros Organics (Thermo Fisher Scientific) (12). The lower limit of quantification was 0.25 μg/ml in plasma and, theoretically, 5 to 15 μg/ml in ELF. The urea method was used to correct for dilution of ELF by PBS in BAL fluid samples, as previously described (12). Then, 5 μl of serum and 20 μl of ELF were used with a QuantiChrom urea assay kit (Gentaur, San Jose, CA) according to the manufacturer's instructions.
The PK parameters (areas under the curve from 0 h to the designated time and from 0 h to infinity [AUC0–t and AUC0–∞], maximum concentration [Cmax], and half-life [t1/2]) were calculated from mean concentrations using Microsoft Excel (Office 2010; Microsoft Corp., Redmond, WA). AUCs were calculated using the linear trapezoidal rule. Half-life and elimination rate constants were calculated by linear regression using semilogarithmic concentration versus time data. Concentrations below the lower limit of quantification were excluded from the PK evaluation.
Pharmacodynamics studies.
To determine the dose-ranging activity of POA administered orally, 140 BALB/c (5 mice per treatment group) and 77 C3HeB/FeJ mice (10 mice per treatment group) received, depending on the study, doses of 37.5, 75, 150, or 450 mg/kg administered QD or doses of 75 or 450 mg/kg administered BID for up to 8 weeks. In rabbits, the infection was allowed to progress for 10 weeks prior to treatment initiation. The remaining rabbits were assigned to control and treatment groups (n = 6). Rabbits in the treatment group received PZA at 175 mg/kg administered orally daily for 5 and 10 weeks.
(i) Assessment of treatment efficacy in mice.
Treatment efficacy was assessed on the basis of lung CFU counts determined after 4 and 8 weeks of treatment. Serial dilutions of whole-lung homogenates were plated on selective Middlebrook 7H11 agar (Becton Dickinson, Franklin Lakes, NJ). Plates were incubated for 6 to 8 weeks at 37°C before the final CFU counts were determined.
(ii) Assessment of selection of POA-resistant mutants in mice.
For all BALB/c mice sacrificed after 8 weeks of treatment, 0.5 ml of lung homogenate was plated directly on selective 7H11 agar enriched with 10% OADC and supplemented with POA at 300 mg/liter. In addition, for C3HeB/FeJ mice with the highest CFU counts after treatment with POA at 75 and 450 mg/kg BID, colonies were scraped from 7H11 plates, homogenized, suspended by bead beating (using 0.1-mm sterile beads) in 2.5 ml of PBS, and subjected to quantitative cultures on the same POA-supplemented 7H11 agar. At the same time, a late-log-phase culture of the parent H37Rv strain in 7H9 broth culture (optical density of 1.09 at 600 nm) was plated on the same POA-containing medium to determine the proportion of spontaneous POA-resistant mutants.
(iii) Assessment of treatment efficacy in rabbits.
Rabbits were sacrificed at 5 and 10 weeks postinfection (four rabbits per group) to monitor the change in bacterial burden prior to the initiation of treatment. Treatment-naive control and treatment groups (six rabbits per group) were sacrificed at 15 and 20 weeks postinfection (after 5 and 10 weeks of treatment, respectively). Upon dissection, the lungs were weighed, and 2 g of tissue from the right and left lungs was collected by random sampling from all lobes of the organ. The tissue sections were homogenized in PBS and serial dilutions were plated on 7H11 agar for CFU enumeration. Plates were incubated for 4 weeks at 37°C before the final CFU counts were determined. These counts and the whole lung weights were then used to calculate the bacterial burden of the entire organ. If no CFU were detected in the undiluted homogenate of either lung, the limit of detection was calculated by assuming 1 CFU in the total volume plated and extrapolating this count to the weight of the whole pair of lungs for each rabbit. For histological studies, five pieces of lung tissue (one per lobe) from each rabbit were fixed in 10% formalin (Thermo Fisher Scientific) and sent to IDEXX BioResearch (Columbia, MO). These sections were embedded in paraffin and used for standard 5-μm sectioning. The sections were then stained with hematoxylin and eosin (H&E) for cellular composition and with acid-fast staining to visualize M. tuberculosis bacilli.
Susceptibility of H37Rv and resistant mutants to POA.
The POA susceptibilities of the parent H37Rv strain, a pncA A146V mutant in the same background selected previously in mice by PZA treatment, and mutants isolated on POA-containing plates in the present experiment were evaluated in 7H9 broth supplemented with 10% OADC, 0.5% Tween, and glycerol that was pH adjusted to 6.8 in a 37°C shaking incubator. The inoculum was prepared by diluting a log-phase broth culture to an optical density at 600 nm of ∼0.1. Tubes containing a range of 2-fold dilutions of POA dissolved in dimethyl sulfoxide and diluted in 7H9 broth were inoculated with 100 μl of this diluted sample and incubated at 37°C. The MIC was defined as the lowest POA concentration that prevented visible growth after 14 days of incubation. For tubes without visible growth after 14 days of incubation, an aliquot of broth was removed, serially diluted, and plated on selective Middlebrook 7H11 agar (Becton Dickinson) to determine viable bacterial counts. The minimum bactericidal concentration (MBC) was defined as the lowest concentration to reduce the initial inoculum by at least 99%. POA MICs were determined in three separate experiments, whereas MBCs were determined in a single experiment.
Data analysis.
Lung CFU counts (x) were log transformed as log10(x + 1) before analysis. Group mean CFU counts after 2 months of treatment were compared using one-way analysis of variance (ANOVA) and Bonferroni's posttest to adjust for multiple comparisons or two-way ANOVA, as appropriate, using GraphPad Prism v.5 (GraphPad Software, San Diego, CA).
RESULTS
Pharmacokinetics of POA in mice after oral administration of PZA or POA. (i) Administration of PZA.
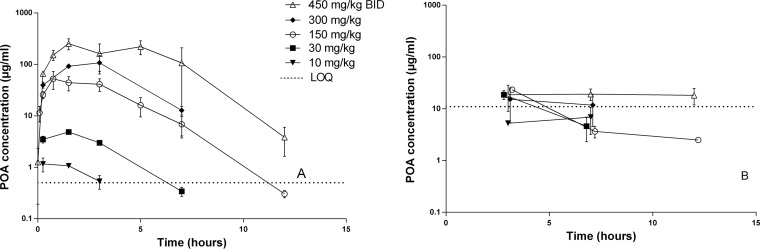
In uninfected animals, PZA doses ranging from 10 to 450 mg/kg were administered as a single dose. Additional mice received 450 mg/kg on two occasions, separated by 12 h. As shown in Fig. 1A, POA plasma concentrations increased with PZA dose. The PK parameter values for plasma presented in Table 1 demonstrate greater than dose-proportional increases in exposure. Data for PZA concentrations in the same samples were published previously (12) but are included here for the sake of comparison. POA AUC0–24 values were consistently one-third to one-half those of PZA. Contrary to PZA exposures in ELF, which increased dose proportionally (12), POA exposures in ELF did not exceed a ceiling of ∼20 μg/ml first observed at 3 h postdose for PZA doses ≥30 mg/kg (Fig. 1B). ELF concentrations fell below this level by 7 h postdose for all mice receiving ≤150 mg/kg and some mice receiving 300 mg/kg, but not mice receiving 450 mg/kg. Remarkably, 3-h ELF POA concentrations exceeded those in plasma after 10- and 30-mg/kg doses but then fell to as low as 14% of plasma concentrations after the 450-mg/kg dose.
FIG 1.
POA concentration-time profiles in plasma (A) and ELF (B) after administration of the indicated dose of PZA to uninfected C3HeB/FeJ mice. Data are plotted as means and SD. LOQ, lower limit of quantification.
TABLE 1.
Plasma PK parameters for PZA and POA after administration of PZA
| Mouse strain infection status | PZA dosing (in mg/kg QD unless specified) | PZA AUC0–∞a (μg · h ml−1) | POA AUC0–∞ (μg · h ml−1) | POA Cmax (μg/ml) | POA t1/2 (h) |
|---|---|---|---|---|---|
| Uninfected C3HeB/FeJ mice (single dose) | 10 | 9.2 | 4.5 | 1.1 | 2.0 |
| 30 | 59.2 | 19.0 | 4.8 | 1.8 | |
| 150 | 577.9 | 222.2 | 52.7 | 1.9 | |
| 300 | 1,081.6 | 534.6 | 107.3 | 3.1 | |
| 450 BID | 5,326.8 | 3,046.6 | 253.3 | 7.0 | |
| Infected C3HeB/FeJ mice (steady state) | 150 | 455.6 | 139.0 | 37.1 | 2.0 |
| 450 BID | 3,589.6 | 1,553.0 | 201.8 | 1.2 | |
| Predicted (from single dose PK)b | 175 | 234 | 581 | ||
| Measured in infected rabbits (steady state) | 175 | 236 | 583 | 68 | 2.3 |
PZA AUCs published previously (12) are included here for the sake of comparison. Parameters were calculated from means of data. Measurements in infected mice were obtained after 4 weeks of treatment. The data presented are from one experiment each.
The pharmacokinetic profile and parameters of PZA after a single oral dose administered to uninfected rabbits as published previously (9).
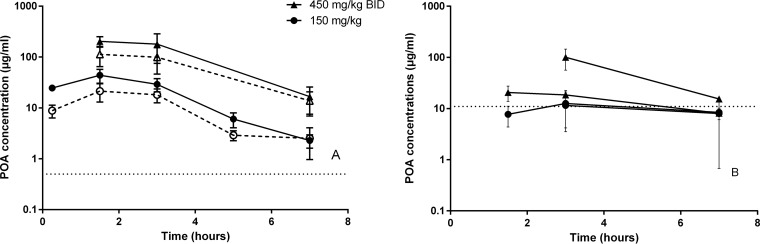
In infected animals, multidose PK was evaluated after administration of 150 mg/kg and 450 mg/kg BID of PZA for 4 weeks. Slightly lower concentrations of POA were found in infected compared to uninfected mice (Table 1 and Fig. 2). Dose proportionality in plasma was conserved, and concentrations in lesions largely mirrored those in plasma. The lesion/plasma AUC ratio was 0.52 and overall independent of dose (0.6 in one experiment and 0.44 in the other). As in uninfected mice, there was no clear evidence of a dose proportional increase in ELF exposure, although in one of the two experiments, the mean ELF POA concentration ranged from 52 to 138 μg/ml at 3 h postdose, making it difficult to conclude whether infection impacts the distribution of POA into ELF at the highest PZA doses. However, the ratios of ELF/plasma concentrations were lower in infected mice than in uninfected animals in the 150 mg/kg arms (between 0.1 and 0.5) and slightly increased over time, but not as much as in uninfected mice (up to 4.2).
FIG 2.
POA concentration-time profiles in plasma and lung lesions (A) and in ELF (B) after administration of the indicated dose of PZA to infected C3HeB/FeJ mice. Open symbols and dotted lines represent intralesional concentrations (A), and solid shapes indicate concentrations in plasma (A) or in ELF (B). Data are plotted as means and were averaged for two experiments in panel A and in exploded view in panel B to show the difference observed. Horizontal dotted lines represent the lower limit of quantification.
(ii) Administration of POA.
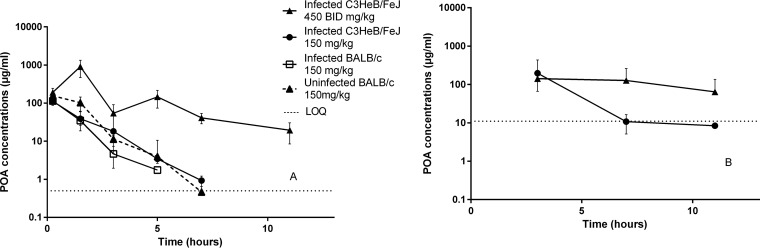
POA was administered as a single dose in uninfected and infected BALB/c mice at 150 mg/kg QD and in infected C3HeB/FeJ mice at 150 mg/kg QD and at 450 mg/kg BID. As observed for POA concentrations after increasing PZA doses, oral administration of increasing doses of POA produced greater than dose-proportional increases in POA exposure in plasma but not in ELF (Fig. 3). The PK parameter values for plasma are presented in Table 2; the plasma AUC was slightly higher in uninfected mice compared to infected BALB/c and C3HeB/FeJ mice, but the differences were relatively modest. Although POA did not exhibit dose proportionality in ELF, POA concentrations were 6 to 8 times higher in the ELF of infected animals administered POA directly compared to POA concentrations after PZA administration, whereas POA concentrations were below the limit of quantification in ELF of uninfected BALB/c mice. The ratios of ELF/plasma concentration again decreased with dose and increased over time. Overall, C3HeB/FeJ mice had higher ratios of ELF/plasma concentrations than infected BALB/c mice (for which only two of nine measurements were above the lower limit of quantification).
FIG 3.
POA concentration-time profiles in plasma (A) and in ELF (B) after administration of the indicated dose of POA. Data are plotted as means. LOQ, lower limit of quantification. ELF concentrations in BALB/c mice are from one mouse only.
TABLE 2.
Plasma PK parameters for POA after administration of POAa
| Mouse strain infection status | PZA dosing (in mg/kg QD unless specified) | AUC0–12 (μg · h ml−1) | AUC0–∞ (μg·h ml−1) | Cmax (μg/ml) | t1/2 (h) |
|---|---|---|---|---|---|
| BALB/c mice (single dose) | |||||
| Uninfected | 150 | 285.9 | 286.5 | 155.3 | 1.0 |
| Infected | 150 | 143.4 | 145.4 | 114.1 | 0.8 |
| Infected C3HeB/FeJ mice (single dose) | 150 | 176.9 | 178.2 | 111.5 | 1.0 |
| 450 BID | 1,965.2 | 4,086.6 | 904.3 | 2.7 |
Parameters were calculated from means of data. The data presented are from one experiment each.
Pharmacodynamics of POA after oral administration. (i) BALB/c mice.
In a subacute infection model, the mean CFU counts (standard deviations [SD]) were 4.03 (0.12) log10 on the day after infection and 7.88 (0.05) log10 at D0. All mice died within 10 days of initiating treatment, indicating the weak inhibitory activity of PZA and POA before the adaptive immune response has developed.
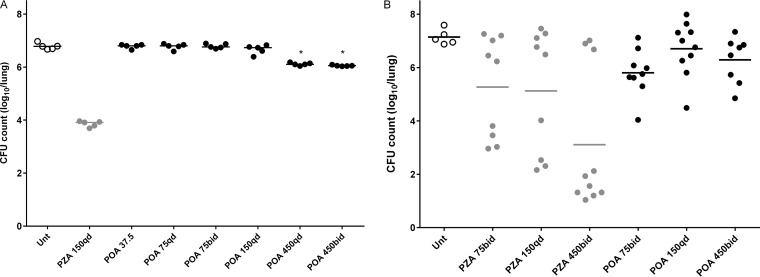
In a chronic model of TB infection in BALB/c mice, the mean CFU counts (SD) were 2.17 (0.05) log10 on the day after implantation and 7.24 (0.04) log10 at the start of treatment (D0). After 4 weeks of treatment, mean CFU counts (SD) were 6.78 (0.12) log10 in untreated mice and 3.85 (0.11) log10 in mice receiving PZA 150 mg/kg. Mean CFU counts of mice treated with total daily POA doses as high as 150 mg/kg did not differ from those of untreated mice. However, mean CFU counts (SD) in mice treated with 450 mg/kg QD and BID were 6.11 (0.05) and 6.05 (0.02) log10, respectively, significantly lower than that of untreated mice (P < 0.001) (Fig. 4A). Similar results were observed after 8 weeks of treatment, when only mice treated with 450 mg/kg QD and BID had mean CFU counts that were statistically significantly lower than that of untreated mice (P < 0.001).
FIG 4.
Individual mouse lung CFU counts after treatment with POA for 4 weeks in BALB/c mice (A) and C3HeB/FeJ mice (B). Bars represent the mean. Doses are provided in mg/kg. qd, once a day; bid, twice a day; Unt, untreated. *, statistically significant difference compared to untreated mice (P < 0.001).
(ii) C3HeB/FeJ mice.
In a chronic model of TB infection in C3HeB/FeJ mice, the mean lung CFU count (SD) was 0.35 (0) log10 on the day after infection and 6.22 (1.00) log10 at the start of treatment (D0). Because of the low implantation, some mice did not develop a productive infection. As a result, all culture-negative lung homogenates after 4 weeks of treatment (five total) were excluded from analysis. As previously observed in the heterogeneous lesions of C3HeB/FeJ mice (12, 13), PZA produced a dichotomous dose-response effect that is dependent on the extent of caseous disease: dose-dependent bactericidal activity was observed in a subset of mice with a lesser extent of disease while no dose response was observed in other mice with large caseous lesions (Fig. 4B). Among the subset of mice experiencing a bactericidal effect of PZA, mean CFU counts (SD) after 4 weeks of treatment were 3.31 (0.4), 2.76 (0.86), and 1.5 (0.39) log10 after treatment with 75 mg/kg BID, 150 mg/kg QD, and 450 mg/kg BID, respectively. The difference between PZA at 150 mg/kg QD and at 75 mg/kg BID was not statistically significant (P = 0.28). Conversely, POA exhibited no clear-cut bactericidal activity. The mean CFU counts (SD) after 4 weeks of treatment were 5.81 (0.87), 6.71 (1.02), and 6.29 (0.86) log10 for 75 mg/kg BID, 150 mg/kg QD, and 450 mg/kg BID, respectively. The lowest CFU counts (4.04 and 4.49 log10) were in two mice treated with either POA 75 mg/kg BID or 150 mg/kg QD, so a bactericidal effect up to that observed at the highest doses in BALB/c mice could not be excluded.
(iii) Drug resistance assessment.
The MIC and MBC of POA against the H37Rv parent strain were 120 to 180 μg/ml and 960 μg/ml, respectively, at pH 6.8 in 7H9 broth. When plated on 7H11 agar with or without 300 μg of POA/ml, the proportion of CFU growing on POA was between 8 × 10−5 and 3 × 10−6 of the total cultivable population of M. tuberculosis H37Rv.
After treatment of C3HeB/FeJ mice with POA for 4 weeks, only a single colony was isolated on POA-containing plates from a mouse given 450 mg/kg BID. The POA MIC for this strain increased to >960 μg/ml, whereas the MIC against the pncA A146V mutant was 180 μg/ml, like the H37Rv parent. After treatment of BALB/c mice with POA for 8 weeks, virtually all mice had POA-resistant strains detectable. However, compared to untreated mice, which harbored POA-resistant colonies at ≤10−6 of the total CFU count, no clear selective amplification was observed in POA-treated mice, since the highest proportion of POA-resistant CFU was 10−5 of the total CFU count (in a mouse treated with POA 450 mg/kg QD). Among PZA-treated mice, a single POA-resistant colony was isolated in a single mouse, which represented 1.7 × 10−4 of the total CFU count, suggesting the potential for PZA to select POA-resistant mutants.
Pharmacodynamics of PZA in rabbits infected with a pncA mutant.
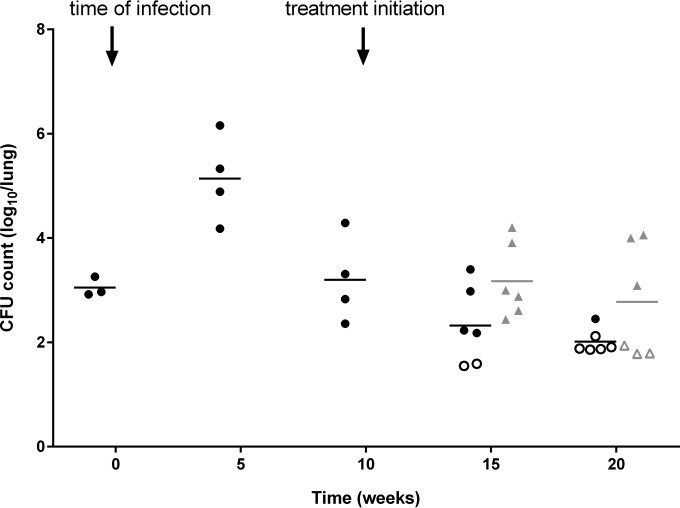
Having determined the poor efficacy of POA delivered orally to mice, we next tested the efficacy of systemic POA produced by the host from orally administered PZA. We previously showed that the POA/PZA exposure ratio is markedly higher in rabbits than in all other species (9) and that the systemic pool of POA distributes effectively to lung and lesions (14), thus providing a convenient tool for assessing the activity of host-derived POA in rabbits infected with a pncA mutant of M. tuberculosis HN878. A daily dose of PZA at 175 mg/kg was selected since it was predicted to deliver a POA AUC0–24 of 581 μg·h/ml (Table 1), corresponding to the high end of the PZA AUC range observed clinically among patients receiving standard PZA doses (15) and higher than the POA AUC recently described in Korean patients receiving PZA (9). The pncA mutant appeared attenuated in its ability to persist relative to historical controls. However, after 5 and 10 weeks of daily PZA treatment (7/7), the bacterial burden was higher in the treated group than in the untreated group (Fig. 5, P = 0.017), indicating that POA treatment had no discernible antimicrobial activity. To ensure that adequate drug exposure had been reached, plasma concentrations were measured at week 6 of treatment, revealing actual PZA and POA AUC PK parameters very close to predicted values (Table 1). Since PZA was previously shown to preferentially target M. tuberculosis bacilli present in cellular lesions (12), histologic analyses were performed, indicating that M. tuberculosis bacilli were present intracellularly in the cellular rim of necrotic lesions, and mostly extracellularly within the caseating core of such lesions (see Fig. S1 in the supplemental material). These results indicate that systemic POA is ineffective against M. tuberculosis bacilli in rabbit lung lesions.
FIG 5.
Mean and individual lung CFU counts of rabbits infected with an M. tuberculosis HN878 pncA-null mutant for 10 weeks, followed by daily treatment with PZA at 175 mg/kg (7 days/week) for 10 weeks. The time of infection and treatment initiation are indicated by arrows. Control groups are represented by circles, and treated groups are represented by triangles. Data points below the lower limit of detection are indicated by open symbols. Treated rabbits had significantly higher CFU counts than untreated rabbits (P = 0.017), as determined by two-way ANOVA.
DISCUSSION
PZA is one of two TB drugs with demonstrated treatment-shortening activity. Addition of PZA to RIF-containing regimens enables shortening of the treatment duration for drug-susceptible TB from 9 months to 6 months (16). Unlike RIF, PZA may also be active against MDR-TB strains, and there is evidence that it helps to shorten the duration of MDR-TB treatment when the infecting isolate is susceptible (17–19). Moreover, PZA combines well with each new TB drug class currently in clinical trials and adds important sterilizing activity to a variety of novel combinations, including the combination of PA-824, moxifloxacin, and PZA, which was recently shown to be at least as effective as the first-line regimen over the first 8 weeks of treatment, including against MDR-TB strains (20–26). Despite its potential importance for short-course MDR-TB treatment now and in the future, the utility of PZA is endangered by the emergence of resistance. Roughly 50% of MDR-TB isolates are now resistant to PZA according to surveys from various parts of the world (27, 28).
Systemic delivery of POA has been suggested as a strategy to circumvent the most common mechanism of PZA resistance. Experiments conducted in mice over 50 years ago failed to demonstrate efficacy of orally administered POA (10, 29). However, these experiments were conducted in an acute infection model in which treatment was initiated within 1 day of an overwhelming infection with Mycobacterium tuberculosis and consisted of POA mixed in the mouse diet. Kushner et al. (29) tested a dose of 0.2% (∼350 to 400 mg/kg) POA in the diet. Under these conditions PZA prevented death (the lone endpoint reported) but POA did not. Konno et al. (10) followed up this observation by using higher doses (2 to 5%), assessing microbiological endpoints and testing for evidence of POA in the urine. While mice receiving PZA had lower lung CFU counts and modestly lower spleen CFU counts compared to untreated controls, mice receiving POA did not. However, much more POA was excreted in the urine of mice receiving PZA compared to POA, suggesting poor bioavailability of POA administered in the diet. Notably, the same investigators gave a 1- to 2-g oral dose of POA to human volunteers and found that, although 62 to 100% of the dose was recovered in the urine, the compound recovered had no microbiological activity (10). As a result of these experiments, it was concluded that POA is not orally bioavailable and/or is too rapidly excreted and/or inactivated in vivo to be effective by this route (or perhaps any systemic administration). This has steered subsequent investigators toward development of new POA derivatives with greater oral bioavailability and intrapulmonary delivery of POA.
The present experiments confirm that POA is well absorbed when administered orally to mice and provide new evidence of greater than dose-proportional exposures in plasma. Importantly, oral doses of POA of 150 and 450 mg/kg produced similar plasma POA AUC values and higher plasma POA Cmax values than the same doses of PZA. Moreover, whereas the POA AUC produced by the 150-mg/kg POA dose in mice was similar to that produced in human subjects after standard doses of PZA, the Cmax and AUC produced in mice by the 450-mg/kg QD and BID doses were significantly higher than systemic POA exposures produced by host-mediated conversion from PZA (9). Likewise, POA derived from PZA metabolism in rabbits reached exposures in excess of clinical exposure following PZA administration but did not reduce bacillary burden. Thus, the limited activity of these systemic POA exposures in vivo suggests that host-derived POA does not significantly contribute to the well-demonstrated sterilizing activity of PZA. To our knowledge, this is the first direct evidence that producing plasma, ELF, and lung lesion POA exposures similar to (or even well in excess of) the POA exposures produced by effective doses of PZA is not sufficient for efficacy. Only the 450-mg/kg dose of POA, administered once or twice daily, produced any statistically significant effect. At these doses, the Cmax and AUC values were each at least 10 times the values obtained through host-mediated conversion of PZA. Based on our PK results, it is possible that prolonged plasma POA concentrations above the MIC at acidic pH (e.g., 30 μg/ml at pH 5.8) are necessary for efficacy. However, such plasma POA concentrations are as high as or higher than peak plasma POA concentrations produced in human subjects by standard doses of PZA. A recent toxicodynamic analysis suggests that prolonged POA concentrations in this range are likely to cause arthralgias and gouty complications (30).
The finding of higher lung CFU counts after treatment with PZA compared to no treatment among rabbits infected with the pncA-null mutant was unexpected. Potential explanations include a detrimental effect on the host response caused by PZA-mediated immunomodulation or the stresses of daily PZA administration by gavages. The failure of the mutant to persist in rabbit lungs in the absence of treatment is also notable. Although pncA is clearly not essential for Mycobacterium bovis to cause disease in rabbits, we are unaware of prior studies on the effect of loss-of-function mutations in pncA on the virulence of M. tuberculosis in rabbits. To better understand the apparent attenuation of the pncA mutant, whole-genome sequencing was performed. In addition to the frameshift mutation in pncA, a single nucleotide polymorphism (Gly367Cys) in ripA, a peptidoglycan hydrolase required for growth in vitro and in vivo (31, 32), was revealed as a potential attenuating mutation. Although we cannot exclude the possibility that ripA mutation confers reduced susceptibility to POA in vivo, the mutant did not display a higher POA MIC in vitro compared to the parent strain. We also have no reason to believe that the attenuation of the mutant produced in vivo conditions that were not conducive to PZA or POA activity, since histologic analyses revealed caseating granulomas containing both intracellular and extracellular bacilli at the time treatment was initiated (10 weeks postinfection), findings indicative of an adaptive host immune response to create acidic conditions sufficient for PZA activity at the concentrations achieved.
Taken together, the results presented here provide strong evidence that, regardless of the vehicle, delivery of POA into the systemic circulation is probably unable to produce efficacious exposures without incurring significant problems with tolerability. A more promising option is to develop prodrugs or other vehicles that liberate POA inside the bacillus (or perhaps within acidified phagosomes harboring bacilli), enabling more efficient concentration of POA in the bacteria (4, 33), while reducing the potential for toxicity. Prodrugs of POA that do not depend on the PncA amidase for release of POA have been described (8, 34–39). One challenge to development of such prodrugs, that is reinforced by the poor activity of systemically administered POA in our study, has been to develop prodrugs that are sufficiently stable in plasma but readily cleaved to release POA inside M. tuberculosis (39).
An alternative or complementary strategy is intrapulmonary delivery of POA or POA prodrugs (36). The apparently poor diffusion of POA into ELF demonstrated here, whether via POA or PZA administration, suggests the possibility of a saturable secretion mechanism. Although the significance of ELF concentrations of POA (or any TB drug, for that matter) remains to be demonstrated, at least one analysis suggests that the accumulation of PZA in ELF is responsible for its initial bactericidal activity in sputum (40). Direct administration of POA or POA prodrugs to the lungs by aerosol delivery might produce higher concentrations of POA at the site of infection while limiting the systemic exposure.
Our in vitro and in vivo experiments indicate that between 1 in 105 and 1 in 106 bacilli in our H37Rv strain are spontaneously resistant to POA at 300 μg/ml at pH 6.8. Given its weak activity in vivo, POA resulted in little or no selective amplification in either mouse strain. This is reassuring given the concern raised about the possibility that continued exposure to host-derived POA could promote additional POA resistance in patients infected with PZA-resistant pncA mutants who receive PZA empirically. Nevertheless, a significant minority of phenotypically PZA-resistant isolates have functional PncA enzymes and may harbor mutations in rpsA, panD, or other previously unidentified mutations conferring resistance to POA (41–43). Thus, further characterization of the POA-resistant isolates obtained here is warranted and is under way.
In conclusion, despite exhibiting good bioavailability and producing systemic POA exposures comparable to, or exceeding, those produced by PZA administration, systemic delivery of POA lacks the efficacy of PZA. More efficient delivery of POA into (or near to) bacilli at the site of infection through new prodrugs metabolized by M. tuberculosis, novel vehicles to deliver a POA payload to infected macrophages, and/or intrapulmonary administration offer greater potential for circumventing the PZA resistance conferred by pncA mutations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Matthew Zimmerman, Firat Kaya, Rada Savic, and Subramanya Lingaraju for help with drug content analysis, dose prediction, and mutant isolation.
This study was funded by the National Institutes of Health through grant R01-AI106398 (V.D.) and a supplement to the Johns Hopkins University Center for AIDS Research P30-AI094189 (E.N.), as well as by the Bill and Melinda Gates Foundation through grants OPP1037174 (E.N.) and OPP1066499 (V.D.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03085-15.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Whitfield MG, Soeters HM, Warren RM, York T, Sampson SL, Streicher EM, van Helden PD, van Rie A. 2015. A global perspective on pyrazinamide resistance: systematic review and meta-analysis. PLoS One 10:e0133869. doi: 10.1371/journal.pone.0133869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KC, Yew WW, Zhang Y. 2011. Pyrazinamide susceptibility testing in Mycobacterium tuberculosis: a systematic review with meta-analyses. Antimicrob Agents Chemother 55:4499–4505. doi: 10.1128/AAC.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 5.Ramirez-Busby SM, Valafar F. 2015. A systematic review of mutations in pyrazinamidase associated with pyrazinamide resistance in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 59:5267–5277. doi: 10.1128/AAC.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Napiórkowska A, Rüsch-Gerdes S, Hillemann D, Richter E, Augustynowicz-Kopeć E. 2014. Characterization of pyrazinamide-resistant Mycobacterium tuberculosis strains isolated in Poland and Germany. Int J Tuberc Lung Dis 18:454–460. doi: 10.5588/ijtld.13.0457. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Nam JS, Kim KJ, Ro YT. 2014. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from Korea and analysis of the correlation between the mutations and pyrazinamidase activity. World J Microbiol Biotechnol 30:2821–2828. doi: 10.1007/s11274-014-1706-0. [DOI] [PubMed] [Google Scholar]
- 8.Speirs RJ, Welch JT, Cynamon MH. 1995. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob Agents Chemother 39:1269–1271. doi: 10.1128/AAC.39.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Via LE, Savic R, Weiner DM, Zimmerman MD, Prideaux B, Irwin SM, Lyon E, O'Brien P, Gopal P, Eum S, Lee M, Lanoix J-P, Dutta NK, Shim T, Cho JS, Kim W, Karakousis PC, Lenaerts A, Nuermberger E, Barry CE, Dartois V. 2015. Host-mediated bioactivation of pyrazinamide: implications for efficacy, resistance, and therapeutic alternatives. ACS Infect Dis 1:203–214. doi: 10.1021/id500028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno K, Feldmann FM, McDermott W. 1967. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis 95:461–469. [DOI] [PubMed] [Google Scholar]
- 11.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanoix J-P, Ioerger T, Ormond A, Kaya F, Sacchettini J, Dartois V, Nuermberger E. 2015. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother 60:735–743. doi: 10.1128/AAC.01370-01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanoix JP, Lenaerts AJ, Nuermberger EL. 2015. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 8:603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prideaux B, Via LE, Zimmerman MD, Eum S, Sarathy J, O'Brien P, Chen C, Kaya F, Weiner DM, Chen PY, Song T, Lee M, Shim TS, Cho JS, Kim W, Cho SN, Olivier KN, Barry CE, Dartois V. 2015. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 21:1223–1227. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu M, Starke JR, Burman WJ, Steiner P, Stambaugh JJ, Ashkin D, Bulpitt AE, Berning SE, Peloquin CA. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686–695. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Leung CC, Yew WW, Leung EC, Leung WM, Tam CM, Zhang Y. 2012. Pyrazinamide may improve fluoroquinolone-based treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother 56:5465–5475. doi: 10.1128/AAC.01300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurbatova EV, Gammino VM, Bayona J, Becerra MC, Danilovitz M, Falzon D, Gelmanova I, Keshavjee S, Leimane V, Mitnick CD, Quelapio MI, Riekstina V, Taylor A, Viiklepp P, Zignol M, Cegielski JP. 2012. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 16:1335–1343. doi: 10.5588/ijtld.11.0811. [DOI] [PubMed] [Google Scholar]
- 19.Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL. 2014. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 20.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim M, Andries K, Lounis N, Chauffour A, Truffot-Pernot C, Jarlier V, Veziris N. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother 51:1011–1015. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, Miller PF. 2011. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother 55:567–574. doi: 10.1128/AAC.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsåker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol 46:3459–3464. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierre-Audigier C, Surcouf C, Cadet-Daniel V, Namouchi A, Heng S, Murray A, Guillard B, Gicquel B. 2012. Fluoroquinolone and pyrazinamide resistance in multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 16:221–223. doi: 10.5588/ijtld.11.0266. [DOI] [PubMed] [Google Scholar]
- 29.Kushner S, Dalalian H, Sanjurjo J, Bach F, Safir S, Smith V, Williams J. 1952. Experimental chemotherapy of tuberculosis. II. The synthesis of pyrazinamides and related compounds. J Am Chem Soc 74:3617–3621. [Google Scholar]
- 30.Pasipanodya J, Gumbo T. 2011. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 55:24–34. doi: 10.1128/AAC.00749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 32.Hett EC, Chao MC, Steyn AJ, Fortune SM, Deng LL, Rubin EJ. 2007. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol Microbiol 66:658–668. doi: 10.1111/j.1365-2958.2007.05945.x. [DOI] [PubMed] [Google Scholar]
- 33.Zimic M, Fuentes P, Gilman RH, Gutiérrez AH, Kirwan D, Sheen P. 2012. Pyrazinoic acid efflux rate in Mycobacterium tuberculosis is a better proxy of pyrazinamide resistance. Tuberculosis 92:84–91. doi: 10.1016/j.tube.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes JP, Pasqualoto KF, Felli VM, Ferreira EI, Brandt CA. 2010. QSAR modeling of a set of pyrazinoate esters as antituberculosis prodrugs. Arch Pharm 343:91–97. [DOI] [PubMed] [Google Scholar]
- 35.Krátký M, Vinšová J, Novotná E, Stolaříková J. 2014. Salicylanilide pyrazinoates inhibit in vitro multidrug-resistant Mycobacterium tuberculosis strains, atypical mycobacteria, and isocitrate lyase. Eur J Pharm Sci 53:1–9. doi: 10.1016/j.ejps.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Durham PG, Zhang Y, German N, Mortensen N, Dhillon J, Mitchison DA, Fourie PB, Hickey AJ. 2015. Spray dried aerosol particles of salts for tuberculosis therapy. Mol Pharm 12:2574–2581. doi: 10.1021/acs.molpharmaceut.5b00118. [DOI] [PubMed] [Google Scholar]
- 37.Cynamon MH, Klemens SP, Chou TS, Gimi RH, Welch JT. 1992. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem 35:1212–1215. doi: 10.1021/jm00085a007. [DOI] [PubMed] [Google Scholar]
- 38.Cynamon MH, Gimi R, Gyenes F, Sharpe CA, Bergmann KE, Han HJ, Gregor LB, Rapolu R, Luciano G, Welch JT. 1995. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J Med Chem 38:3902–3907. doi: 10.1021/jm00020a003. [DOI] [PubMed] [Google Scholar]
- 39.Simões MF, Valente E, Gómez MJ, Anes E, Constantino L. 2009. Lipophilic pyrazinoic acid amide and ester prodrugs stability, activation, and activity against Mycobacterium tuberculosis. Eur J Pharm Sci 37:257–263. doi: 10.1016/j.ejps.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi W, Chen J, Feng J, Cui P, Zhang S, Weng X, Zhang W, Zhang Y. 2014. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis. Emerg Microbes Infect 3:e58. doi: 10.1038/emi.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dillon NA, Peterson ND, Rosen BC, Baughn AD. 2014. Pantothenate and pantetheine antagonize the antitubercular activity of pyrazinamide. Antimicrob Agents Chemother 58:7258–7263. doi: 10.1128/AAC.04028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.