Abstract
Background
Recent studies in experimental mice have shown that mild deficiency of methylenetetrahydrofolate reductase (MTHFR) enzyme confers protection against malaria, thus providing an important basis for the hypothesis that MTHFR polymorphism, i.e. C677T, might have been subjected to selection pressure against malaria. The present study was undertaken in a malaria endemic region in North East India to assess whether a similar selection advantage exists for other genes in folate metabolism pathway.
Methods
A total of 401 subjects including 131 symptomatic malaria, 97 asymptomatic malaria and 173 normal healthy controls were analysed for nine polymorphisms (single-nucleotide polymorphisms [SNPs] in eight genes and insertion/deletion in one gene): MTHFR C677T, methionine synthase reductase (MTRR) A66G, glutamate carboxypeptidase II (GCPII) C1561T, cystathionine beta-synthase (CBS) 844ins68, reduced folate carrier-1 (RFC-1) G80A, serine hydroxymethyltransferase (SHMT) C1420T, methionine synthase (MTR) A2756G, MTHFR G1793A (D 919G), glycine N-methyltransferase (GNMT) 1289 by PCR-RFLP technique. Differences in frequencies of genotype distribution of each polymorphic marker between these groups were evaluated.
Results
MTRR A2756G, SHMT C1420T, GCPII C1561T, MTRR A2756G and GNMT C1289T and RFC1 G80A polymorphisms showed significantly different prevalence between different groups analyzed. No significant differences were seen in the distribution of other polymorphisms.
Conclusions
The study gives a clue for the possible selection of specific polymorphisms in the genes involved in the folate metabolism pathway by malaria parasite.
Keywords: Folate metabolism pathway polymorphisms, India, Malaria, MTHFR, Selection
Introduction
The major cause of mortality among children worldwide is malaria, which annually kills more than 1 million children in Africa alone and is estimated to cause about half a billion episodes of disease each year due to Plasmodium falciparum infection only.1 Malaria is unique in that it has exerted the strongest known selective pressure to the human genome.2 The classic example is HbS allele which has risen due to the high frequencies in malaria exposed populations, despite the fact that homozygotes have high mortality.3 The different geographic distribution of α thalassemia, G6PD deficiency and Duffy negative blood groups are a few other examples of the general principle that different populations have selected different polymorphisms to protect from malaria parasite.3,4
All mammalians require folate metabolism to recycle methionine and homocysteine. Malarial parasites are capable of de novo folate synthesis and they also have hydrofolate reductase which is more sensitive to antimalarial inhibitors than that of the host cell.5 There are also reports which suggest that breakdown products of folate may be used by parasites for this de novo synthesis.6 Thus it is possible that if the parasite has restricted folate cycle, as mentioned above, it might depend on more complete host cell folate cycle to carry out other reactions such as metabolism of increased levels of homocysteine produced by methionine use. When there is concurrent occurrence of methylenetetrahydrofolate reductase (MTHFR) mutation, folate/vitamin B6/B12 deficiency combined with high methionine use by malarial parasite, there may be acute imbalance in the pathway.7 The elevated level of homocysteine during acute P. falciparum infection suggests that the balance in the folate cycle is disturbed, which could be a consequence of the reduced availability of vitamin B12, caused by increased oxidative stress.8 This may suggest a selection for the C677T MTHFR allele, driven by P. falciparum in many parts of the world.9
MTHFR is an important one regulatory enzyme in the folate and homocysteine metabolism pathway besides being involved in nucleotide synthesis and remethylation reactions. The enzyme acts as a catalyst in the reduction of 5,10-methylenetetrahydrofolate to produce 5-methyltetrahydrofolate, which in turn acts as the methyl donor for the remethylation of homocysteine to methionine.10,11 MTHFR deficiency has been found to be also associated with increased risk of myocardial infarction, cancers, inherited bleeding disorders and neural tube defects and Down syndrome.12–15
The common polymorphism, i.e., MTHFR C677T (rs1801133:C>T) which is associated with mild deficiency of the enzyme, persists with high prevalence in different populations even in homozygous state.16 A recent study in experimental mice has clearly shown that mild MTHFR deficiency protects from malaria which provides further evidence for the hypothesis that this polymorphism has been subjected to selection pressure in malaria endemic regions.17
Not many studies are available on the general prevalence of other single nucleotide polymorphisms (SNPs) in the genes involved in folate metabolism pathway. The disease association studies with the variants of folate metabolism are also few, but it is possible that gene-gene interactions may exist among the SNPs in the folate metabolism pathway. Cumulative effect of these SNPs have been reported in cases of Down syndrome and neural tube defects.18,19
A large number of malaria infected cases in North East India have been found to be asymptomatic for several years despite high parasitemia. The genetic basis of resistance is complex at several levels. It is likely that many genes are involved and they interact with the environmental variables and with parasitic genetic factors. Susceptibility and resistance to malaria can only be studied in regions of high transmission of malaria, where one is repeatedly bitten by infected mosquitoes resulting in diverse clinical manifestations.20
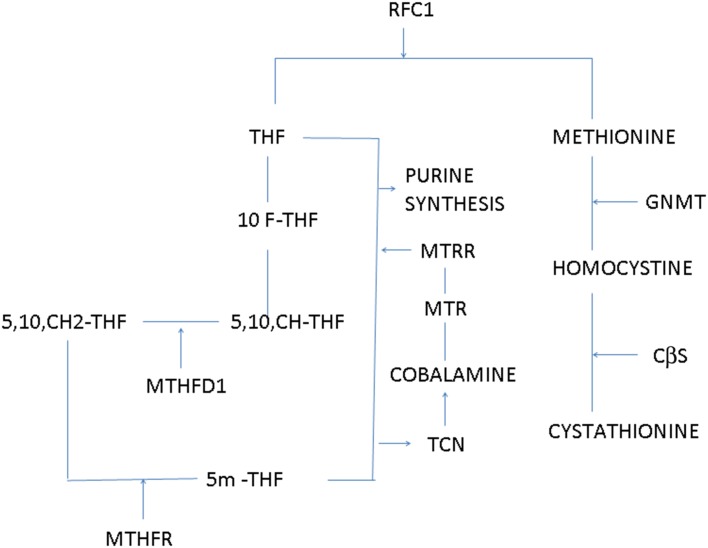
Figure 1 shows the role of different enzymes involved in the folate metabolism. The present study is an attempt to propose whether there is any difference in the prevalence of the polymorphisms in the folate metabolism pathway in three different groups (symptomatic malaria, asymptomatic malaria and normal healthy subjects) from a malaria endemic region in North East Indi. .
Figure 1.
Role of different enzymes in folate metabolism pathway. 10F-THF: 10-formyltetrahydrofolate; 5,10, CH-THF: 5,10-methylenetetrahydrofolate; CβS: cystathionine beta-synthase; GNMT: glycine N-methyltransferase; MTHFR: methylenetetrahydrofolate reductase; MTHFRD1: methylenetetrahydrofolate dehydrogenase; MTR: methionine synthase; MTRR: methionine synthase reductase; RFC1: reduced folate carrier-1; TCN: transcobalamin; THF: tetrahydrofolate.
Materials and methods
Patients and controls
After obtaining approval from the ethics committees of both participating institutes, 5 cc of blood was collected, from consenting normal and malaria infected patients, in ethylenediaminetetraacetic acid (EDTA) at the Regional Medical Research Centre (RMRC), Dibrugarh, India. The blood samples were collected between February 2012 and December 2014. The thick and thin smears were prepared immediately and the remaining blood sample was frozen at −20°C till it was shipped to Mumbai for DNA analysis. The clinical details were taken in a well-designed proforma which included demographic details like age, sex and geographic location, time of onset of symptoms and diagnosis, past history of infection, and treatment. Subjects analyzed were as follows: 97 asymptomatic, with microscopically confirmed Plasmodium infection but without any clinical symptoms (chills, headache, generalized body and joint pains) and 131 symptomatic with positive parasitemia and presenting with classical symptoms of malaria infection. Also included were 173 age and sex matched normal healthy controls, who had never had any malaria infection from the same geographical region and who were negative for malaria parasites under light microscopy. Both thick and thin films were stained with 10% Giemsa and examined by two expert microscopists in the laboratory. If no asexual parasites were observed in 200 high power fields, the patient was considered negative for malaria infection at the time of presentation. The percentage parasitemia was calculated by counting the number of parasitised red cells in 1000 cells in a thin blood film. The percentage calculation of parasitemia in a thin blood film was expressed by the number of infected cells as a percentage of the red blood cells, i.e., three parasitized red cells/100 red blood cells or 3% parasitemia.21 The asymptomatic malaria samples are the samples which were collected from the relatives of malaria patients and who claimed that they had never had malaria. The malaria parasitemia status and density were determined under oil immersion with the 100x objective of a light microscope.22
Polymorphism analysis
Genomic DNA was extracted from whole blood using commercial kits (Qiagen, Hilden, Germany). SNPs and insertion-deletion were analyzed using previously reported primers19,23–25 (Sigma Aldrich, St. Louis, MO, USA) (Supplementary Table 1) with or without restriction enzyme digestion. All PCR amplifications were carried out in GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). The amplified products were run on 2% agarose gel (Invitrogen, Bengaluru, India). Wherever required, PCR products were digested with specific restriction enzymes (Fermentas, Baltimore MD, USA) (Supplementary Table 1). The digested products were analysed by 10% polyacrylamide gel electrophoresis (PAGE).
Statistical analysis
The data was analyzed using MedCalc Statistical online software version 15.8 (MedCalc Software bvba, Ostend, Belgium). The differences in prevalence were analyzed using the Fishers exact test. A difference with a p-value ≤0.05 was considered statistically significant.
Results
A total of 401 samples which included 131 symptomatic, 97 asymptomatic malaria and 173 normal healthy controls from the same geographical region were tested for folate metabolism pathway related polymorphisms.
When symptomatic and asymptomatic malaria patients were compared, three polymorphisms i.e. serine hydroxymethyltransferase (SHMT) C1420T, methionine synthase reductase (MTRR) A2756G and glycine N-methyltransferase (GNMT) C1289T were found to be significantly different between the two groups(p<0.05). When compared between patients with malaria and normal controls, SHMT C1420T, MTRR A2756G, GNMT C1289T were still significantly different between the two groups. In addition, MTRR A66G, reduced folate carrier-1 (RFC-1) G80A and cystathionine β-synthase (CBS) 844ins68 polymorphisms were different between the two groups (Table 1). About one-third of the controls had the RFC1 G80A polymorphism which was not found in any of the patients with malaria. Table 1 shows the distribution of the genotypes and alleles in different groups.
Table 1.
Polymorphisms analyzed in the present study
| Polymorphisms | Variants | Asymptomatic patients | Symptomatic patients | p-value | Controls | Total malaria patients | p-value |
|---|---|---|---|---|---|---|---|
| MTHFR C677T | CC | 72 | 99 | NS | 127 | 171 | NS |
| CT | 23 | 32 | 43 | 55 | |||
| TT | 2 | 0 | 3 | 2 | |||
| MTRR 66AG | AA | 20 | 34 | NS | 66 | 54 | 0.0019 |
| AG | 77 | 97 | 98 | 174 | <0.0001 | ||
| GG | 0 | 0 | 9 | 0 | 0.0244 | ||
| GCP II C1561T | CC | 97 | 131 | NS | 168 | 228 | NS |
| CT | 0 | 0 | 5 | 0 | |||
| TT | 0 | 0 | 0 | 0 | |||
| CβS 844ins68 | Insertion negative | 92 | 121 | NS | 173 | 213 | 0.0251 |
| Insertion positive | 5 | 10 | 0 | 15 | 0.0251 | ||
| RFC I G80A | GG | 24 | 44 | NS | 10 | 68 | <0.0001 |
| GA | 73 | 87 | 105 | 160 | 0.0475 | ||
| AA | 0 | 0 | 58 | 0 | 0.0001 | ||
| SHMT C1420T | CC | 24 | 16 | 0.0156 | 89 | 40 | <0.0001 |
| CT | 73 | 115 | 0.0156 | 84 | 188 | <0.0001 | |
| TT | 0 | 0 | NS | 0 | 0 | NS | |
| MTRR A2756G | AA | 10 | 79 | <0.0001 | 146 | 89 | 0.0001 |
| AG | 78 | 43 | <0.0001 | 27 | 121 | <0.0001 | |
| GG | 9 | 9 | 0.3794 | 0 | 18 | 0.0174 | |
| MTHFR G1793A | GG | 69 | 94 | NS | 128 | 163 | NS |
| GA | 28 | 37 | 45 | 65 | |||
| AA | 0 | 0 | 0 | 0 | |||
| GNMT C1289T | CC | 44 | 78 | 0.0344 | 89 | 122 | NS |
| CT | 31 | 24 | 0.0185 | 21 | 55 | 0.0029 | |
| TT | 22 | 29 | NS | 63 | 51 | 0.0022 |
CβS: cystathionine beta-synthase; GNMT: glycine N-methyltransferase; MTHFR: methylenetetrahydrofolate reductase; MTR: methionine synthase; MTRR: methionine synthase reductase; NS: not significant; RFC1: reduced folate carrier-1.
Discussion
This is the first report of a comprehensive analysis of folate metabolism pathway polymorphisms in association with malaria infection. Despite several eradication programmes across the globe, malaria still continues to prevail causing an estimated 560 000 deaths per year, mostly children under 5 years of age. In malaria endemic regions, total eradication has still not been possible due to several reasons, the most important being the presence of patients with asymptomatic malaria, who do not seek medical treatment.26 The presence of higher incidence of asymptomatic malaria in any part of the world is a big challenge and a serious concern for malaria control programmes; it may serve as a reservoir for continued transmission, and may further complicate diagnosis.
Asymptomatic malaria is a known phenomenon in malaria-endemic areas with high transmission.27 It is associated with low parasite densities, usually observed in patients later in life living in high endemic areas after repeated infections of malaria and has been attributed to different factors, some of which include development of acquired immunity to P. falciparum.28
There are reports to show that host parasite interaction plays a major role in the selection of malaria parasite during evolution.29 Host factors also play an important role in this selection process. For instance, erythrocytes are protected from increased infection by the malarial parasite through the up regulation of a hormone, i.e., hepcidin.30 Hepcidin induction during infection causes depletion of extracellular iron, which is presumably a general defence mechanism against infections by withholding iron from invading parasites. This implies that the host parasite interaction will be altered if the host iron levels are altered.
The interaction between folate metabolism and malaria parasite is very well known. Among the folate pathway polymorphisms, MTHFR C677T polymorphism has been extensively investigated for its association with various pathogenic conditions including recurrent fetal loss, Down syndrome and neural tube defects.12–15 In a recent study, MTHFR deficient mice and MTHFR over expressing mice were infected with Plasmodium berghei ANKA to induce cerebral malaria. MTHFR mice survived longer than MTHFR overexpressing mice.17 A similar result was obtained earlier in the knockout mice (Mthfr−/− mice) to cytomegalovirus.31 The data clearly suggests that mild MTHFR deficiency protects against severe malaria and that this phenomenon may have led to the high frequency of the C677T variant in human populations across the world.
In the present study MTRR A2756G polymorphism showed significantly different prevalence between the asymptomatic (80.4%, 78/97) and symptomatic (32.8%, 43/131) malaria groups and between malaria patients and controls (p=0.0001). Surprisingly, the RFC-1 G80A homozygotes were absent in malaria patients (0%) as compared to one-third of the normal healthy controls (33.5%, 58/173). RFC-1 is important in folate metabolism and it helps in active transport of 5-methyltetrahydrofolate from the plasma to the cytosol. It also facilitates movement of folate and thiamine monophosphate across the cell membrane, thus maintaining an optimum level of folate both within and outside the cells.32 Polymorphisms in this gene are known to affect its binding with folate.33 In addition, RFC-1 also plays an important role in folate homeostasis where it gets down-regulated in response to folate deficiency.34 The gene is highly polymorphic and has been found to be associated with different diseases like ischemic stroke, neural tube defects and different types of cancers.
The study has several limitations. First, no phenotype-genotype association could be made in the present study. Second, the sample size is small in each group to draw a definitive conclusion. Third, molecular tools were not used to detect low density parasitemia in both asymptomatic patients and normal healthy controls. Despite these limitations, the study highlights the need for extension of these studies to other populations in malaria endemic regions in this country.
Conclusions
In conclusion, the genotype frequencies of SHMT C1420T, MTRR A2756G and GNMT C1289T were found significantly different between symptomatic and asymptomatic malaria and between patients with malaria and normal healthy controls in the same geographical region. The MTRR A66G, RFC1 G80A and CBS 844ins68 polymorphisms also showed significantly different prevalences between normal controls and patients with malaria. The RFC-1 G80A polymorphism was totally absent in patients with malaria as compared to one-third of normal controls. Whether these polymorphisms were subjected to a selection pressure similar to MTHFR will only be confirmed in large studies in malaria endemic regions.
Supplementary data
Supplementary data are available at Transactions online (http://trstmh.oxfordjournals.org/).
Acknowledgments
Authors’ contributions: MD performed the laboratory experiments and analyzed the data; MJ, DP performed the clinical examination of the patients; GK, SS and MJ designed the study and wrote the manuscript. All authors read and approved the final manuscript. SS is guarantor of the paper.
Funding: The project was funded by Department of science and technology (DST) [Grant No. HS 108/2011].
Competing interests: None declared.
Ethical approval: The project was approved by institutional ethics committee of both National Institute of Immunohematology, Mumbai and Regional Medical Research Centre, Dibrugar.
References
- 1.Snow RW, Gurerra CA, Noor AM et al. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005;434:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tishkoff SA, Williams SM.. Genetic analysis of African polulation human evolution and complex disease. Nat Rev Gen 2002;3:611–21. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski Dominic P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 2005;77:171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tournamilli C, Colon Y, Carttron JP et al. Description of a GATA motif gene expression in Duffy negative individuals. Nat Genet 1995;10:224–8. [DOI] [PubMed] [Google Scholar]
- 5.Beagli B, Yang TL, Hung J et al. The Glysin 1289 C-T variant influences plasma total homocysteine concentrations on young women after restricting folate intake. J Nutr 2005;135:2780–5. [DOI] [PubMed] [Google Scholar]
- 6.Dittrich S, Mitchell SL, Blagborough AM et al. An atypical orthologue of 6-pyruvoyltetrahydropterin synthase can provide the missing link in the folate biosynthesis pathway of malaria parasites. J Nutr 2008;67:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebbring SJ, Chai Y, Ji Y et al. Serine hydroxymethyltransferase 1 and 2: gene sequence variation and functional genomic characterization. J Neurochem 2012;120:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji Y, Nordgren KK, Chai Y et al. Human liver methionine cycle: MAT1A and GNMT gene resequencing, functional genomics, and hepatic genotype-phenotype correlation. Drug Metab Dispos 2012;40:1984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chillemi R, Zappacosta B, Simporè J et al. Hyperhomocysteinemia in acute Plasmodium falciparum malaria: an effect of host-parasite interaction. Clin Chim Acta 2004;348:113–20. [DOI] [PubMed] [Google Scholar]
- 10.Locke AE, Dooley KJ, Tinker SW et al. Variation in folate pathway genes contributes to risk of congenital heart defects among individuals with Down Syndrome. Genet Epidemiol 2010;34:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TT, Dyer DL, Dunning DD et al. Human intestinal folate transport: cloning, expression, and distribution of complementary RNA. Gastroenterology 1997;112:783–91. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Stampfer MJ, Ma J et al. Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis 2001;154:667–72. [DOI] [PubMed] [Google Scholar]
- 13.Sharp L, Little J.. Polymorphism in genes involved in folate metabolism and colorectal neoplasia: A HuGE review. Am J Epidemiol 2004;159;423–4. [DOI] [PubMed] [Google Scholar]
- 14.Wilson A, Platt R, Wu Q et al. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab 1999;67:317–23. [DOI] [PubMed] [Google Scholar]
- 15.O'Leavy VB, Parle-McDermott A, Molloy AM et al. MTRR and MTHFR polymorphisms, link to Down syndrome. Am J Med Genet 2002;107:151–5. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Sánchez C, Gómez-Gutierrez A, Gómez PE et al. C677T (RS1801133 ) MTHFR gene polymorphism frequency in a Colombian population. Colomb Med (Cali) 2015;46:75–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Meadows DN, Pyzik M, Wu Q et al. Increased resistance to malaria in mice methylenetetrahydrofolate reductase (MTHFR) deficiency suggests a mechanism for selection of the MTHFR 677C>T(c.665C>T) variant. Hum Mutat 2014;35:594–600. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs CA, Sherman SL, Yi P et al. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet 2000;67:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relton CL, Wilding CS, Pearce MS et al. Gene-gene interaction in folate-related genes and risk of neural tube defects in a UK population. J Med Genet 2004;41:256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doolan Denise L., Carlota Doban, Kevin Baird J.. Acquired immunity to malaria. Clin Microbiol Rev 2009;22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manser M, Olufsen C, Andrews N, Chiodini PL.. Estimating the parasitaemia of Plasmodium falciparum: experience from a national EQA scheme. Malar J 2013;12:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apinjoh TO, Anchang-Kimbi JK, Njua-Yafi C et al. Association of candidate gene polymorphisms and TGF-beta/IL-10 levels with malaria in three regions of Cameroon: a case-control study. Malar J 2014;13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aifan Li, Hong Zheng, Yuming Xu et al. The association between gene polymorphisms of homocysteine metabolism-related enzymes and ischemic cerebrovascular diseases in Chinese Henan Han population. Life Sci J 2012;9:403–8. [Google Scholar]
- 24.Bonsch D, Bayerlein K, Reulbach U et al. Different allele-distribution of mthfr 677 C -> T and mthfr -393 C ->a in patients classified according to subtypes of Lesch's typology. Alcohol Alcohol 2006;41:364–7. [DOI] [PubMed] [Google Scholar]
- 25.Beagle B, Yang TL, Hung J et al. The glycine N-methyltransferase (GNMT) 1289 C->T variant influences plasma total homocysteine concentrations in young women after restricting folate intake. J Nutr 2005;135:2780–5. [DOI] [PubMed] [Google Scholar]
- 26.Lindblade KA, Steinhardt L, Samuels A et al. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 2013;11:623–39. [DOI] [PubMed] [Google Scholar]
- 27.Nkoghe D, Akue JP, Gonzalez JP, Leroy EM.. Prevalence of Plasmodium falciparum infection in asymptomatic rural Gabonese populations. Malar J 2011;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steenkeste N, Rogers WO, Okell L et al. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province Cambodia: implication for malaria elimination. Malar J 2010;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lelliott PM, McMorran BJ, Foote SJ, Burgio G.. The influence of host genetics on erythrocytes and malaria infection: is there therapeutic potential. Malar J 2015;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portugal S, Carret C, Recker M et al. Host-mediated regulation of superinfection in malaria. Nat Med 2011;17:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fodil-Cornu N, Kozij N, Wu Q et al. Methylenetetrahydrofolate reductase (MTHFR) deficiency enhances resistance against cytomegalovirus infection. Genes Immun 2009;10:662–6. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Diop-Bove N, Visentin M, Goldman ID.. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 2011;31:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chango A, Emery-Fillon N, de Courcy GP et al. A polymorphism (80G->A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metab 2000;70:310–5. [DOI] [PubMed] [Google Scholar]
- 34.Ifergan I, Jansen G, Assaraf YG.. The reduced folate carrier (RFC) is cytotoxic to cells under conditions of severe folate deprivation. RFC as a double edged sword in folate homeostasis. J Biol Chem 2008;283:20687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]