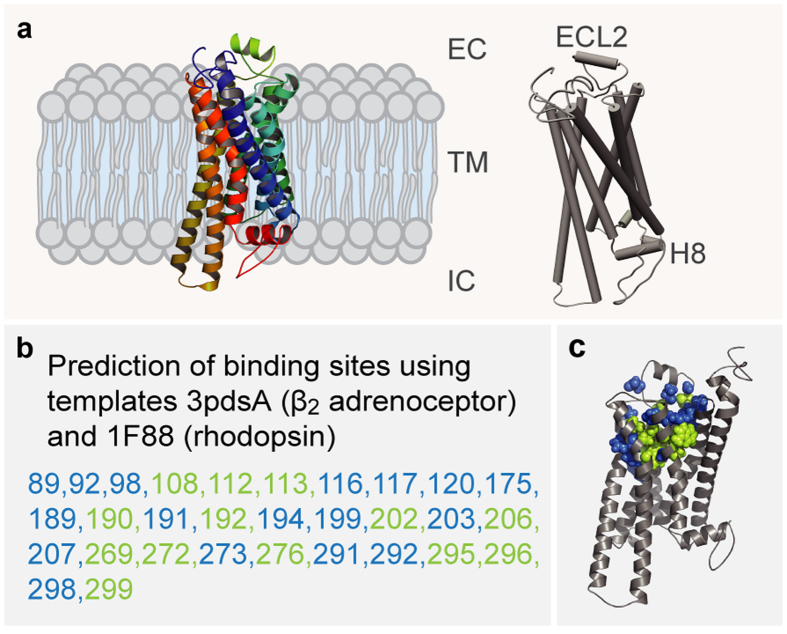
Figure 1. Homology modeling of TAAR13c predicts 7 TM, two additional short helices, and potential binding residues clustered in the upper third of the TM region.
(a) Cartoon representation of the TAAR13c model based on comparison with six crystal structures shows the expected seven transmembrane domains and two short extra helices. The planks representation to the right shows a short α-helix to be located in ECL2 and an intracellular eighth helix, H8 located parallel to the membrane plane. (b) Ligand binding residues (given as residue number in TAAR13c) as predicted by sequence profile comparison with binding sites of PDB templates 3pdsA (FAUC-50-β2 adrenoceptor complex) and 1F88 (bovine rhodopsin); blue, residues only reported in one of the models; green, residues predicted in both models. (c) Predicted binding residues listed in panel (b) shown as spheres in the TAAR13c structure, color code as before. Note the presence of two ‘green’ columns.