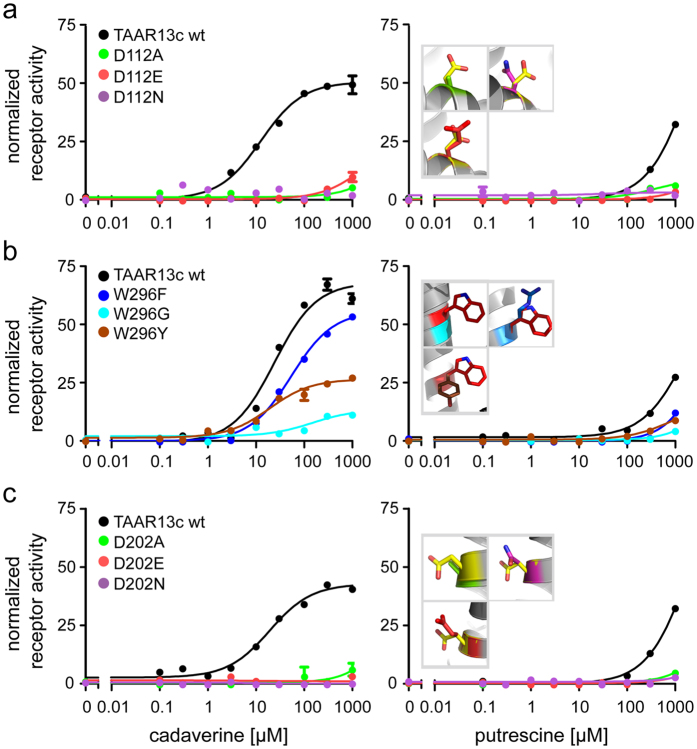
Figure 3.
Concentration-response curves of TAAR13c mutated in the internal binding site and Trp2967.40. HEK293 cell lines constitutively expressing CNG channels and either wildtype or TAAR13c mutants were incubated with concentration series of cadaverine or putrescine (10 nM to 1 mM). Changes in intracellular Ca2+ were detected by Fluo-4 and calculated as ∆F/F. Values were normalized to the fluorescence ratio obtained with NKH477, an agonist of membrane-bound adenylyl cyclases. Representative binding curves are shown color-coded for mutation (green, A; red, E; magenta, N; blue, F; cyan, G; brown, Y), error bars (SD, n = 4) are shown if exceeding symbol size. Left column, responses to cadaverine; right column, responses to putrescine. Insets in right panels show the positions of the mutant side chains (same color as for the respective binding curves) overlayed over the wildtype residue (D, yellow and W, red, respectively). (a) Mutation of the D112 residue to D112E, D112N and D112A results in almost complete loss of activation by cadaverine, with small residual activity only at the highest concentration tested, 1mM. Activation by putrescine is abolished in all D112 mutants. (b) Mutants W296F, W296Y and even W296G were activated by both cadaverine and putrescine, at similar EC50’s as wildtype TAAR13c. Efficacy for W296F is very similar to wildtype TAAR13c but reduced for W296Y and W296G. (c) Mutation of the D202 residue to D202E, D202N, and D202A results in complete loss of activation by cadaverine and putrescine.