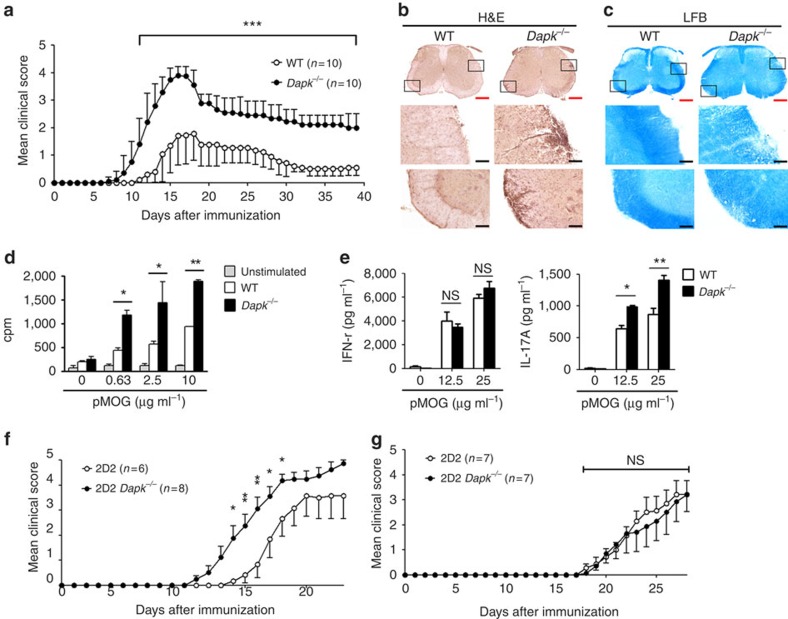
Figure 1. DAPK deficiency increases T-cell activation and EAE exacerbation.
(a) EAE induction in wild-type (WT) and Dapk−/− mice. DAPK knockout and WT control mice were immunized with 200 μg of myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 emulsified in CFA, followed by intraperitoneal injection of 200 ng pertussis toxin at day 0 and day 2. The progression of disease was monitored. Values are mean±s.d. n=10 for each group. (b,c) Increased mononuclear cell infiltration and demyelination in spinal cords from primed Dapk−/− mice. Spinal cords from WT and Dapk−/− mice were isolated 11 days after MOG immunization, and fixed and frozen sections were obtained. Tissue sections were stained with hematoxylin and eosin (H&E) (b) and luxol fast blue (LFB) (c). Red scale bar, 400 μm; black scale bar, 80 μm. Photos are representative of three mice in each group. (d) Enhanced T-cell response to MOG (33–55) in Dapk−/− mice. Splenic CD4 T cells were harvested 9 days after immunization. Cells were stimulated with irradiated autologous presenting cells plus MOG peptide, and the incorporation of thymidine was determined 72 h later. (e) Increased IL-17 production due to MOG (33–55) stimulation in Dapk−/− T cells. The secretion of IFN-γ and IL-17 was quantitated in T cells from d. Data (d,e) are mean±s.d. (n=3), and are representative of three independent experiments. (f) 2D2 Dapk−/− Th17 cells induced exacerbated EAE in Rag1−/− mice. CD4+ T cells from 2D2 or 2D2 Dapk−/− mice were differentiated into Th17 cells in vitro. 2D2 or 2D2 Dapk−/− Th17 cells were re-stimulated and transferred intravenously to Rag1−/− mice, followed by intraperitoneal administration of pertussis toxin. Mice were monitored for clinical signs of paralysis and recorded daily. (g) Comparable EAE generation by 2D2 WT Th1 cells and 2D2 Dapk−/− Th1 cells in Rag1−/− mice. 2D2 or 2D2 Dapk−/− Th1 cells were transferred to Rag1−/− mice, and clinical signs were monitored, as described in f. Values are mean±s.e.m. (f,g). *P<0.05, **P<0.01, ***P<0.001 for unpaired t-test. NS, not significant.