Fig. 1.
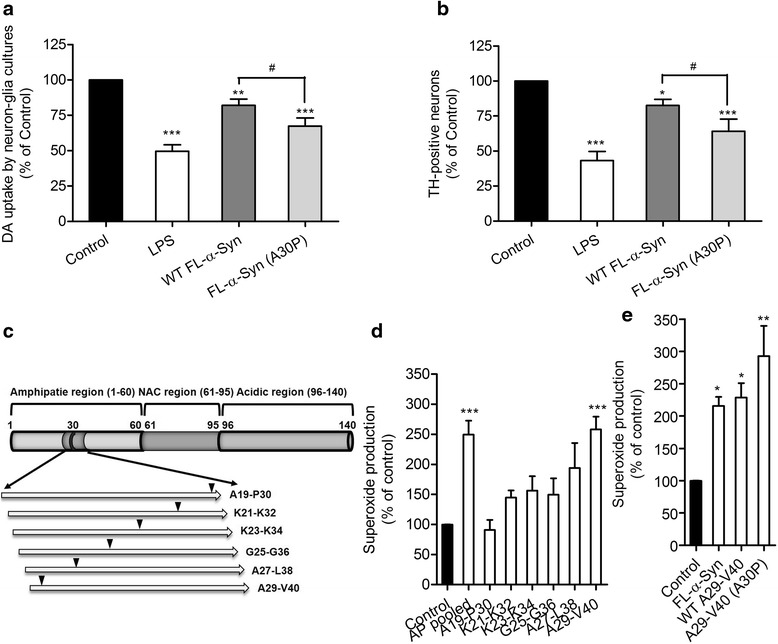
Synthesis of α-Syn peptides and production of microglial superoxide (O 2 • −) upon stimulation of full-length α-Syn (FL-α-Syn) or its peptides. a Changes in the ability of rat neuron-glia cultures to take up 3H-labeled DA after exposure to WT FL-α-Syn aggregates, their corresponding mutant A30P, or LPS which serves as an experimental control. b Changes in the number of TH-positive neurons in rat neuron-glia cultures after exposure to WT FL-α-Syn aggregates, their corresponding mutant A30P, or LPS. c A schematic diagram illustrating the structure of full-length α-Syn (FL-α-Syn) and the strategy for α-Syn peptide synthesis. The location of the A30P point mutation is marked by an arrow. d Levels of extracellular superoxide in mouse microglia-enriched cultures upon the stimulation of BSA (control) or raw α-Syn peptides in which each peptide has a purity of approximately 75 % and harbors the point mutation A30P. e Based on the results collected from the screening test in b, highly purified AP71 peptide, i.e., A29-V40 (A30P), and its corresponding WT, namely A29-V40 (purity >98 %), were selected to further stimulate mouse microglia. Production of extracellular superoxide was measured. BSA treatment serves as a basal control; in contrast, FL-α-Syn aggregates act as a positive control. In d, e, ANOVA analysis plus Newman-Keuls multiple comparisons were performed. *P < 0.05, **P < 0.01, and ***P < 0.001, compared to the BSA basal controls
