Fig. 6.
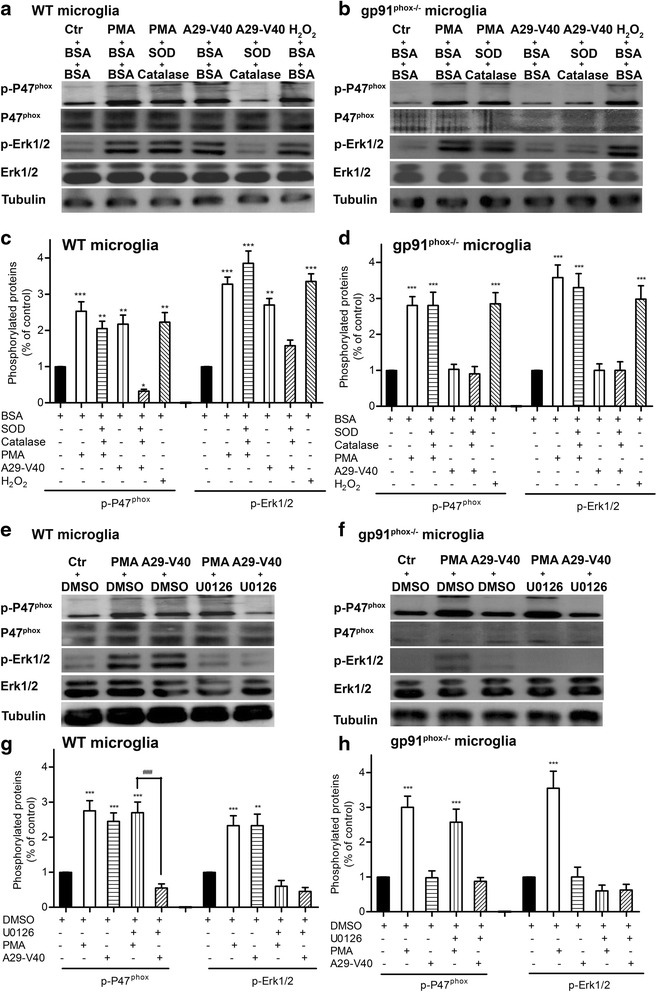
Exposure to A29-V40 peptide activates Erk1/2 to phosphorylate p47phox and to amplify Nox2 activation, dependent on the presence of H2O2. a Effect of the presence of SOD and catalase on the phosphorylation of WT microglial p47phox and Erk1/2. WT mouse microglia were seeded on six-well plates and pretreated with or without SOD and catalase. After stimulation by PMA, α-Syn peptide, or H2O2 in the presence or absence of SOD and catalase, cells were lysed in IP buffer containing protease inhibitors. Cell lysates were subject to SDS-PAGE and were immunoblotted for p47phox, phosphorylated p47phox, Erk1/2, phosphorylated Erk1/2, or tubulin. b Effect of the presence of SOD and catalase on the phosphorylation of p47phox and Erk1/2 in gp91phox null microglia. Microglia deficient in gp91phox were seeded on six-well plates and pretreated with or without SOD and catalase. After stimulation by PMA, α-Syn peptide, or H2O2 in the presence or absence of SOD and catalase, cells were lysed in IP buffer containing protease inhibitors. Cell lysates were subject to SDS-PAGE and were immunoblotted for p47phox, phosphorylated p47phox, Erk1/2, phosphorylated Erk1/2, or tubulin. c Quantitative analysis of the phosphorylation of p47phox and Erk1/2 in WT microglia based on the immunoblot data shown in a. d Quantitative analysis of the phosphorylation of p47phox and Erk1/2 in gp91phox null microglia based on the immunoblot data shown in b. e Effect of the presence of Erk inhibitor U0126 on the phosphorylation of WT microglial p47phox and Erk1/2. WT mouse microglia were seeded on six-well plates and pretreated with or without U0126. After stimulation by PMA or α-Syn peptide in the presence or absence of U0126, cells were lysed in IP buffer containing protease inhibitors. Cell lysates were subject to SDS-PAGE and were immunoblotted for p47phox, phosphorylated p47phox, Erk1/2, phosphorylated Erk1/2, or tubulin. f Effect of the presence of Erk inhibitor U0126 on the phosphorylation of p47phox and Erk1/2 in gp91 null microglia. Microglia deficient in gp91phox were seeded on six-well plates and pretreated with or without U0126. After stimulation by PMA or α-Syn peptide in the presence or absence of U0126, cells were lysed in IP buffer containing protease inhibitors. Cell lysates were subject to SDS-PAGE and were immunoblotted for p47phox, phosphorylated p47phox, Erk1/2, phosphorylated Erk1/2, or tubulin. g Quantitative analysis of the phosphorylation of WT microglial p47phox and Erk1/2 based on the immunoblot data shown in e. h Quantitative analysis of the phosphorylation of p47phox and Erk1/2 in gp91phox null microglia based on the immunoblot data shown in f. In c, d, g, and h, ANOVA analyses followed by Newman-Keuls multiple comparison tests were performed. n = 3–4; *P < 0.05, **P < 0.01, and ***P < 0.001, compared with the corresponding controls. ### P < 0.001, compared as indicated
