Fig. 8.
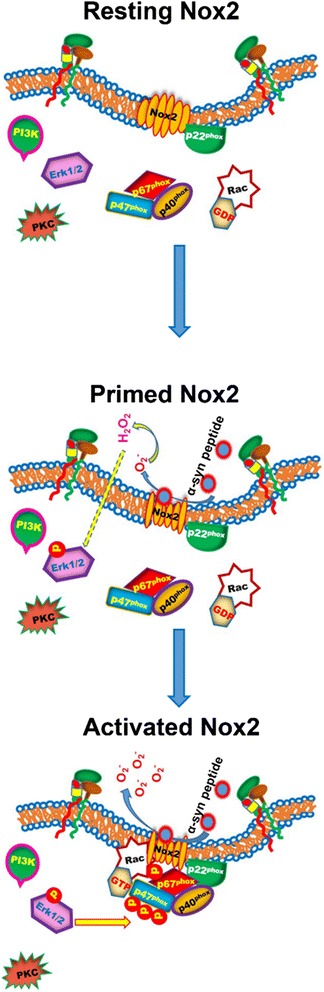
A schematic of the mechanisms by which A29-V40 peptide activates Nox2. The Nox2 catalytic unit gp91phox is located on cell membranes together with the p22phox unit. In a resting status, gp91phox retains an inactivated conformation, and other components of Nox2 including p47phox, p67phox, p40phox, and small GTPase Rac stay in the cytosol. When exposed to the A29-V40 α-Syn peptide (or similar peptides with critical amino acid sequence), microglial gp91phox is bound to this peptide, which possibly initiates a conformational change in gp91phox and directly primes Nox2 to produce O2 • −. Superoxide (O2 • −) is readily converted to H2O2, either spontaneously or catalyzed by SOD. As a cell membrane-permeable chemical, H2O2 is able to diffuse into cytosol, oxidizing cysteine residues in the protein kinase Src and activating it. This process further activates the Ras-Raf-MEK-ERK signal transduction pathway to phosphorylate p47phox and p67phox. As a result, Nox2 activity is further enhanced and more superoxide ions are produced, establishing a positive feedback loop to amplify Nox2 activation. This response includes (1) an “outside to inside” step which begins with the binding of A29-V40 α-Syn peptide and gp91phox and leads to activation of the Ras-Raf-MEK-ERK signal transduction pathway; (2) an “inside to outside” step which is referred to Erk1/2-induced p47phox and p67phox phosphorylation and the ensuing amplified Nox2 activation
