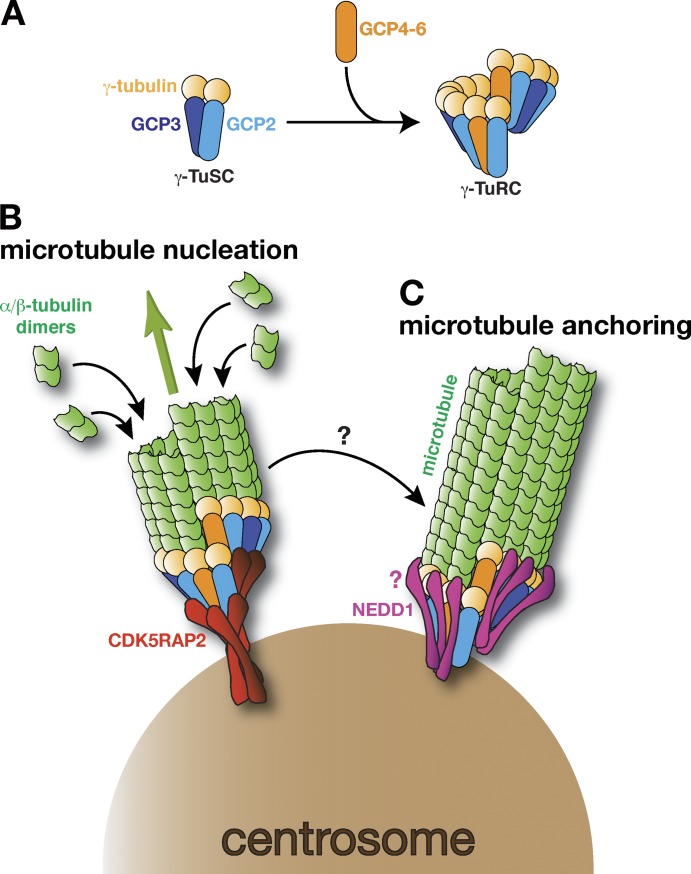
Figure 1.
γ-TuRCs bound to different tethering proteins have different functions in mouse keratinocytes. (A) A schematic of a γ-TuSC comprising γ-tubulin, GCP2, and GCP3, and the larger γ-TuRC, which includes GCP4–6. During γ-TuRC formation, γ-TuSCs associate laterally into helical ring structures and it is thought that some of the GCP2/3 molecules are replaced with GCP4–6. How other non-GCPs associate with γ-TuRCs is not known and is not depicted here. (B) A schematic of a γ-TuRC bound by CDK5RAP2 (red), which is catalyzing (green arrow) the formation of a new microtubule (green) from the centrosome (brown). This process is called microtubule nucleation. (C) A schematic of a γ-TuRC bound by NEDD1 (pink), which is anchoring a microtubule at the centrosome. It is likely that the CM1 domain located in the N terminus of CDK5RAP2 binds directly to the GCP ring, but how NEDD1 binds to the γ-TuRC and how this allows the γ-TuRC to remain associated with (i.e., anchor) the microtubule remains unclear (denoted by the pink question mark). Whether a microtubule nucleated by a CDK5RAP2-bound γ-TuRC is transferred to a NEDD1-bound γ-TuRC also remains unclear (as denoted by the black arrow with a question mark).