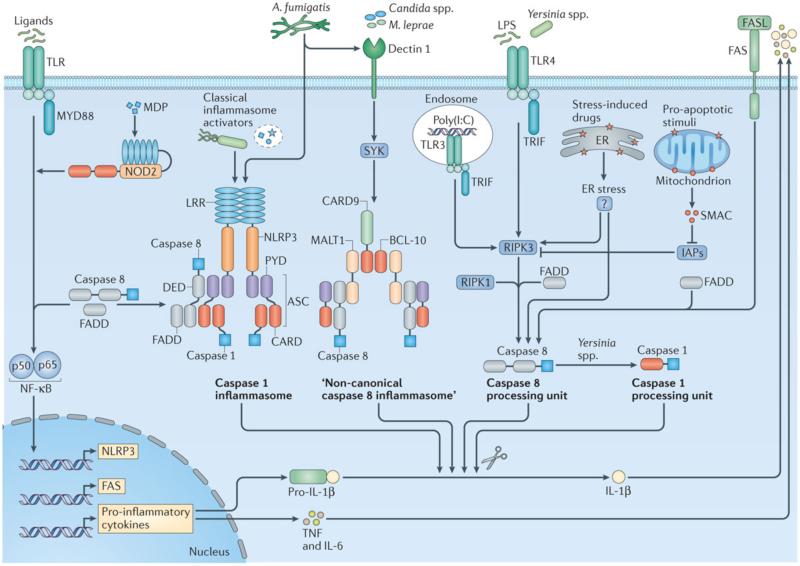
Figure 3. Caspase 8 mediates initiation of NF-κB signalling and maturation of IL-1β.
Recognition of ligands by Toll-like receptors (TLRs) leads to signalling via the adaptor MyD88 and subsequent NF-κB activation. Caspase 8 and its adaptor Fas-associated death domain (FADD) contribute to this pathway, inducing transcription of genes encoding pro-inflammatory cytokines, some of which such as pro-IL-1β and pro-IL-18 require further proteolytic processing 93-100. Caspase 8 or FADD is also recruited to the NLRC4, NLRP3 and AIM2 inflammasome complexes activated by classical inflammasome activators (Salmonella enterica serovar Typhimurium, LPS plus ATP or nigericin, Citrobacter rodentium, Escherichia coli, Francisella novicida, dsDNA, or Aspergillus fumigatus), in which caspase 8 either activates caspase 1, leading to caspase 1-dependent processing of pro-IL-1β and pro-IL-18, and/or modulates cell death 95, 96, 111, 112, 117. However, in the absence of caspase 1, caspase 8 may induce apoptosis in the inflammasome speck 112, 113. Caspase 8 also assembles a range of structurally diverse complexes to directly mediate processing of pro-IL-1β independently of caspase 1. Candida species, A. fumigatus and Mycobacterium leprae infection trigger dectin 1 and its adaptor SYK to engage a so-called “non-canonical caspase 8 inflammasome” complex that is composed of CARD9, BCL-10, MALT1, ASC and caspase 8. Caspase 8 directly processes pro-IL-1β in the absence of caspase 1 and does not require internationalization of the pathogen 104. In response to TLR3 or TLR4 activators, the adaptor TIR-domain-containing adaptor protein inducing IFNβ (TRIF) promotes assembly of a caspase 8 processing unit for direct cleavage of IL-1β in a manner that may require the kinases RIPK1 and RIPK3, and the adaptor FADD 101, 105-108. For example, TLR4-TRIF signalling via LPS and a yet-unidentified signal released as a result of stress in the endoplasmic reticulum (ER) engage caspase 8-dependent processing of IL-1β with or without RIPK3 (Ref 108). LPS-induced TLR4-TRIF signalling and release of second mitochondrial-derived activator of caspases (SMAC; also known as DIABLO) owing to exposure to proapoptotic signals delivered by doxorubicin, staurosporine or cycloheximide activate caspase 8-dependent processing of IL-1β 106, 107. Here, SMAC or SMAC mimetics suppress inhibitor of apoptosis proteins (IAPs) from blocking RIPK3. During Yersinia infection, RIPK1 and FADD are essential in mediating caspase 8 activation via TRIF- and RIPK3-dependent and -independent mechanisms, whereby activation of caspase 8 ultimately drives caspase 1-mediated processing of IL-1β 115, 116. Signalling via TLRs induces upregulation of FAS (also known as CD95 and TNFRSF6). Interaction between FAS and FASL licenses caspase 8-dependent IL-1β release via FADD, independently of RIPK3 or caspase 1 (Ref 109, 110).