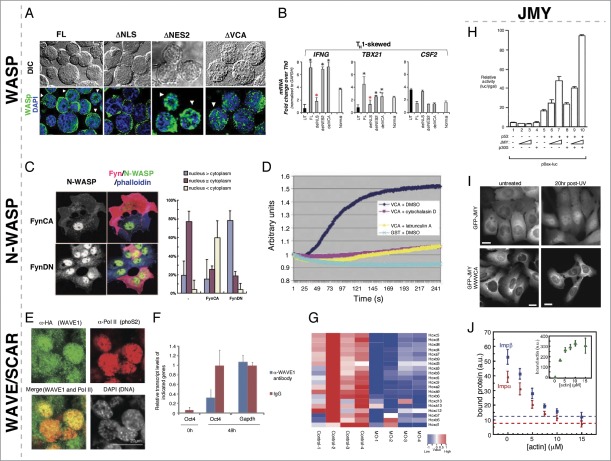
Figure 2.
Nuclear roles for WASP/N-WASP, WAVE/SCAR, and JMY. (A) WASP nuclear localization is regulated by canonical NLS and NES import/export. De-convolved fluorescence micrographs of TH1-skewed WASPNULL cells rescued with the indicated WASP constructs. Expression from the full-length and ΔVCA constructs is found in both the nucleus and cytoplasm, whereas the ΔNLS is only found in the cytoplasm and the ΔNES2 is only found in the nucleus. (B) Nuclear-localized WASP in TH1-skewed cells is responsible for expression of the TH1 regulators IFNG and TBX21. Bar plot graph of gene expression quantitated by RT-qPCR in TH1 WASPNULL (UT) and rescued by full-length (FL), ΔNLS (delNLS), ΔNES2 (delNES2), and ΔVCA (delVCA) constructs compared to TH0 expression levels showing that the TH1 factors IFNG and TBX21 are not-regulated properly in ΔNLS TH1 skewed cells. CSF2 is a non-TH1-specific control. (C) Phosphorylation of N-WASP by the Src kinase, Fyn, leads to enhanced cytoplasmic and decreased nuclear accumulation of N-WASP. Fluorescent micrographs of COS-7 cells expressing ectopic constitutively active Fyn (FynCA) or dominant negative (FynDN) immunostained for anti-Fyn (red), anti-N-WASP (gray/green), and Phalloidin (blue) show that FynCA leads to more cytoplasmic N-WASP, whereas FynDN leads to more nuclear N-WASP. Quantification of N-WASP nuclear versus cytoplasmic N-WASP in COS-7 cells expressing FynCA and FynDN. (D) N-WASP can polymerize actin from nuclear lysates. Pyrene actin assay showing that a GST fusion to the N-WASP VCA domain can polymerize actin, however, it is unable to do so in the presence of the actin polymerization inhibitors cytochalasin D and Latrunculin A. (E) Wave1 is present in mouse C2C12 nuclei after transplantation into the germinal vesicle of Xenopus oocytes. Mouse somatic C2C12 nuclei were transplanted into Xenopus oocytes overexpressing HA-NLS-WAVE1. Immunofluorescence staining for anti-HA (WAVE1) showed WAVE1 co-localizing with active RNA polymerase II in these transplanted nuclei 24 hrs post-nuclear transfer. (F) Nuclear Wave1 is required for transcriptional reprogramming in Xenopus oocytes. Transcriptional activation of the embryonic gene, Oct4, is inhibited in the presence of antibodies against WAVE1 in transplanted somatic nuclei, as measured by QPCR. Expression of the housekeeping gene, Gapdh, was unaffected by the presence of α-WAVE1 antibodies. (G) Nuclear Wave1 is required for hox gene expression. Heat map showing the down-regulation of hox gene expression in WAVE1-morpholino (MO) injected embryos relative to control. (H) JMY and p300 function together to regulate p53-dependent transcription. Bax promoter-luciferase reporter assays were used to measure p53-dependent transcriptional activity. Co-expression of p53 with increasing levels of JMY resulted in a titratable increase in p53 activity that was further enhanced by the expression of p300. (I) The JMY NLS is required for damage-induced nuclear accumulation. Cells expressing GFP-JMY, but not a GFP-JMY truncation mutant removing its actin binding and NLS (GFP-JMYΔWWWCA), show accumulation of GFP signal in the nucleus in response to UV irradiation induced DNA damage. (J) Actin competes with Impα/β for binding to JMY. Actin and importins both bind to a C-terminal fragment of JMY containing tandem WH2 (WWW) motifs and a nested NLS sequence (WWWCA). Quantification of GST pulldowns assaying Impα/β binding to the GST-WWWCA in the presence of increasing concentrations of actin monomers. Permissions. (A–B) Reprinted from Sadhukan et al. (2014).36 The Journal of Immunology 193:150-60. (C) Reprinted with permission from Suetsugu & Takenawa, J. Biol. Chem. 278(43):42515-23.37 © The American Society for Biochemistry and Molecular Biology. Reproduced by permission of The American Society for Biochemistry and Molecular Biology. Permission to reuse must be obtained from the rightsholder. (D) © Macmillan Publishers Ltd: Nature Cell Biology. Reproduced by permission of Macmillan Publishers Ltd: Nature Cell Biology. Permission to reuse must be obtained from the rightsholder. Wu et al., Nat. Cell Biol. 8(7):756–63.40 (E–G) © AAAS. Reproduced by permission of AAAS. Permission to reuse must be obtained from the rightsholder. From Miyamoto et al. (2013) Science 341(6149):1002-5.45 (H) © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder. Reprinted from Molecular Cell, Volume 4(3), Shikama et al., A Novel Cofactor for p300 that Regulates the p53 Response, pp. 365-376.46 (I-J) © American Society for Cell Biology. Reproduced by permission of American Society for Cell Biology. Permission to reuse must be obtained from the rightsholder. From Actin binding to WH2 domains regulates nuclear import of the multifunctional actin regulator JMY, Zuchero et al., Mol. Biol. Cell 23:853, 2012.47