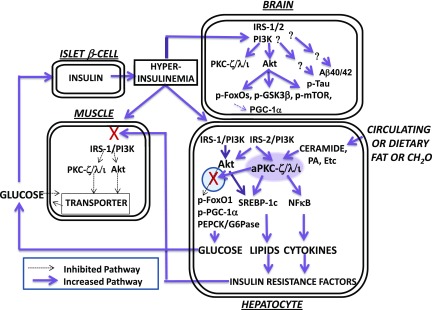
Figure 8.
Hyperactivation of insulin-dependent signaling factors in brain of mice with DIO and systemic insulin resistance. Note that lipid- and carbohydrate-rich diets provoke increases in hepatic ceramide, which potently activates hepatic aPKC, which thereupon selectively inhibits hepatic Akt-dependent FOXO1 phosphorylation within a subcellular compartment (defined by the presence of scaffolding protein WD40/ProF) shared by Akt and aPKC. As a result, there are increases in hepatic FOXO1 and PGC-1α activity and subsequent expression and abundance of PGC-1α and gluconeogenic enzymes, PEPCK and G6Pase. With increased release of glucose from liver, hyperinsulinemia ensues and stimulates “open” (unblocked) insulin-regulated pathways. Thus, in early stages (as depicted here and further reviewed in the study by Sajan et al. [16]), hyperinsulinemia provokes increases in hepatic Akt activity and further increases in hepatic aPKC activity. These increases in the activities of hepatic Akt and aPKC, in turn, provoke increases in the expression and abundance of hepatic lipogenic enzymes and proinflammatory factors, and these, and/or other liver-derived factors, impair insulin signaling to IRS-1, IRS-1–dependent phosphatidylinositol 3-kinase (PI3K), Akt, and aPKC in muscle, thereby impairing glucose transport and glycogen synthesis therein. In later stages (not depicted here), the activation of hepatic IRS-1, IRS-1–dependent PI3K, and Akt diminishes (also secondary to, or abetted by, excessive aPKC activity), but, in contrast, the activation of hepatic IRS-2 and IRS-2–dependent PI3K remains intact and contributes to continued hyperactivation of hepatic aPKC. In human hepatocytes, these abnormalities are further heightened by the fact that the expression and abundance of the primate-specific aPKC, PKC-ι, is strongly autostimulated by aPKC itself through a positive-feedback loop that is intensified by insulin and ceramide, thus creating a vicious cycle within the liver. As a result of hyperinsulinemia, the activities of Akt and aPKC are increased in brain, and phosphorylation of FOXO family members and other Akt substrates are similarly increased. As a result of increases in brain FOXO phosphorylation, FOXO activity is diminished, and this leads to decreases in brain PGC-1α activity and levels. Tau phosphorylation is also increased in brains of insulin-resistant mice and monkeys, but the responsible protein kinase remains uncertain. Although not depicted here (but described in the text), tissue-selective inhibition of hepatic aPKC can reverse abnormalities in liver, muscle, and brain. NFκB, nuclear factor-κB; PA, phosphatidic acid.