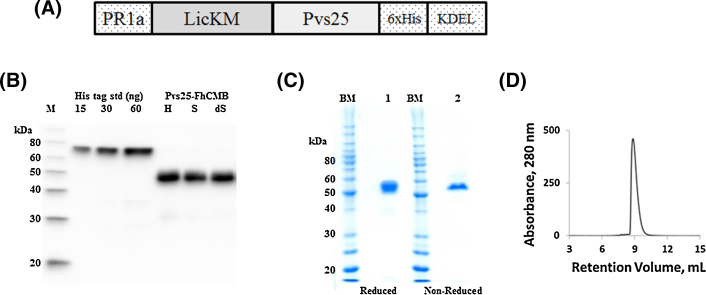
Fig. 1.
Design, expression and purification of Pvs25-FhCMB.
(a) Schematic representation of the Pvs25-FhCMB expression construct showing positions of the PR-1a leader sequence, LicKM carrier protein, Pvs25 antigen and C-terminal 6xHis tag and KDEL. (b) Western blot showing expression of Pvs25-FhCMB in unclarified homogenate (H), soluble (S) and detergent-solubilized (dS) fractions. Molecular weight markers (M) are MagicMark standards (Invitrogen). (c) SDS-PAGE (10%) analysis of Pvs25-FhCMB (2 μg load) stained with Coomassie. Pvs25-FhCMB was run under denatured, reducing conditions (1) or denatured, non-reducing conditions (2). Molecular weight markers (M) are BenchMark standards (Invitrogen). (d) Analytical SEC (Zenix 300) of purified Pvs25-FhCMB (200 μg load).