Figure 7.
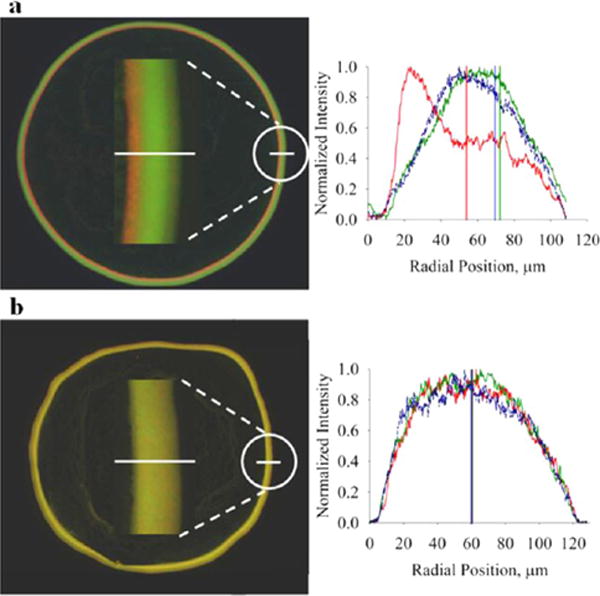
Particle density alters the final coffee ring structure in dried drops. Ring structures of dried, 1 μL water drops containing a mixture of different density particles imaged by fluorescence microscopy: (a) 1:2:1 mixture of PS–COOH (red), MF–COOH (green), and silica (blue) and (b) equal fractions of PS–COOH (red), PS–COOH (green), and silica (blue). Fluorescence profiles for the corresponding white line through the ring structure show that radial particle organization at the ring is partly determined by the densities of constituent particles. Red PS–COOH particles are shifted to the left in a because a greater fraction of these particles is trapped in the air–water interface compared to the MF–COOH and Si particles, causing them to enter the ring structure at later times compared to MF–COOH and Si particles. When the MF–COOH particle is replaced by a PS–COOH particle (green), all particles enter the ring at approximately the same time (b).
