Figure 3.
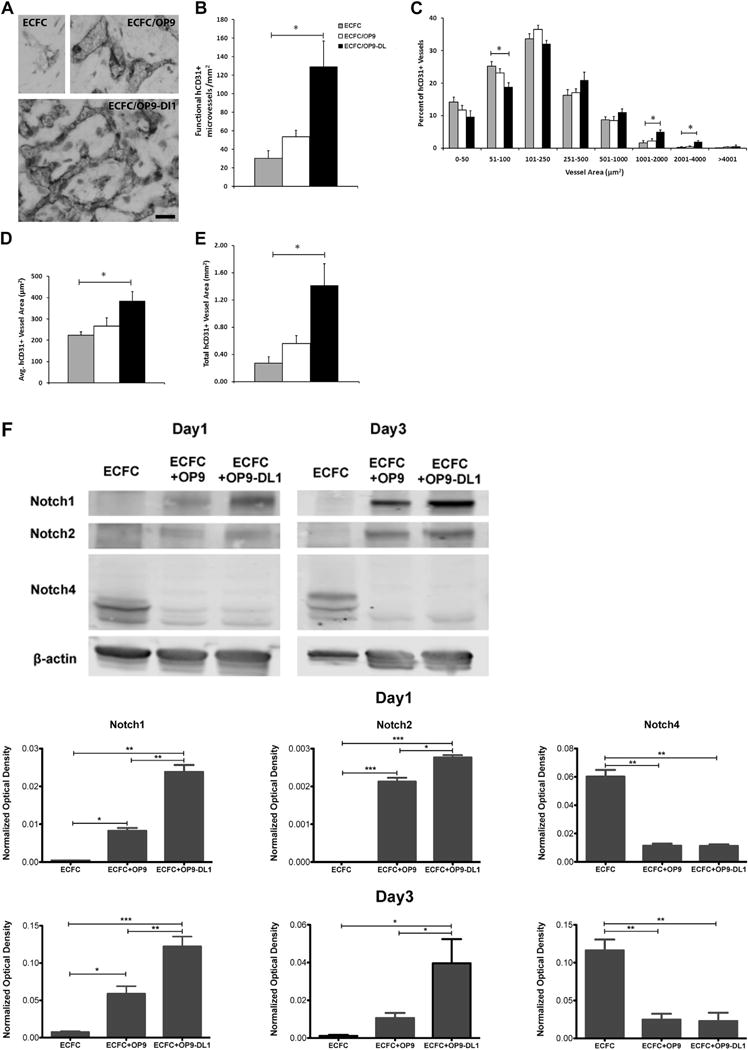
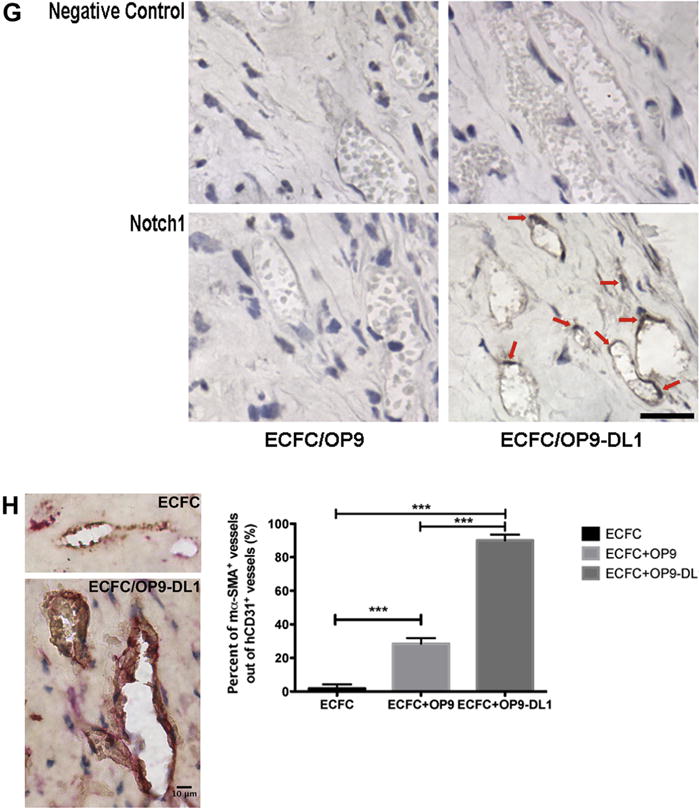
In vivo Dll1 stimulation boosts the formation of functional vessels (n = 3). (A) Anti-human CD31 staining identified human CB–derived ECFCs, alone or combined with OP9 or OP9-DL1, that have formed microvessels in collagen gels after 14 days of implantation. Upon the stimulation of Dll1, there was a significant increase in the number of vessels formed by human CB–derived ECFCs and perfused with murine red blood cells per mm2 in the gel (B). In addition, the size distribution of hCD31+ microvessels was noticeably altered (C), shifting toward larger-sized vessels (1001–4000 μm2) with Dll1 stimulation. Moreover, vessel morphology was significantly altered by the presence of Dll1 with increased average vessel area (D) and total vascular areas (E). (F) Upper panel shows representative immunoblots of Notch 1, Notch 2 and Notch 4 proteins and lower 2 panels show quantification of repeated experiments (protein expression levels were normalized using β-actin); n = 3. *P < 0.05. Anti-cleaved Notch 1 (Val1744) antibody staining (G) in endothelial nuclei confirms that the activation of Notch 1 is detected in vivo in newly formed human vessels in the presence of Dll1; n = 6. Scale bar represents 100 μm. (H) Anti-mouse smooth muscle α actin (αSMA) antibody staining (red) was detected in perivascular cells around human ECFC–derived CD31+ vessels (brown) in implants with OP9-DL1 stromal cells. Scale bar represents 10 μm. *P < 0.05; **P < 0.001; ***P < 0.0001.
