FIGURE 2.
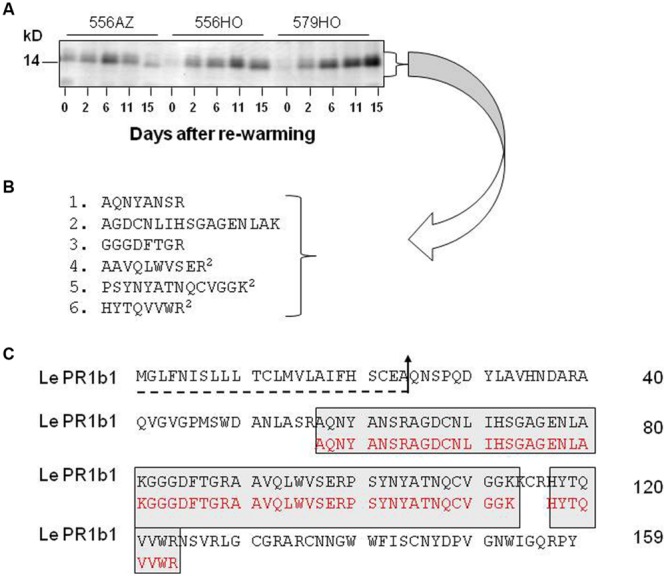
The chilling-induced ∼14 kD protein is PR1b1. (A) Shown is a Coomassie-blue R-250 stained SDS-PAGE gel (Supplementary Figure S1) strip around the ∼14 kD region following fractionation of total soluble protein from samples taken on the days shown after re-warming the chilled fruits at 20°C (14-d chilled fruit is designated by day 0). Each lane contained equal amount of protein. (B) Micro sequencing analysis of chilling-induced ∼14 kD protein. The protein was purified, eluted and trypsinized and amino acid sequence of tryptic-peptides was determined by MALDI-MS. The superscripts indicate the times those particular peptides were re-sequenced. (C) Alignment of the protein amino acid sequence (lower lanes in red) with deduced amino acid sequence of tomato PR1b1 (upper lanes). The dotted line with arrow shows the putative cleavage site of hydrophobic signal peptide.
