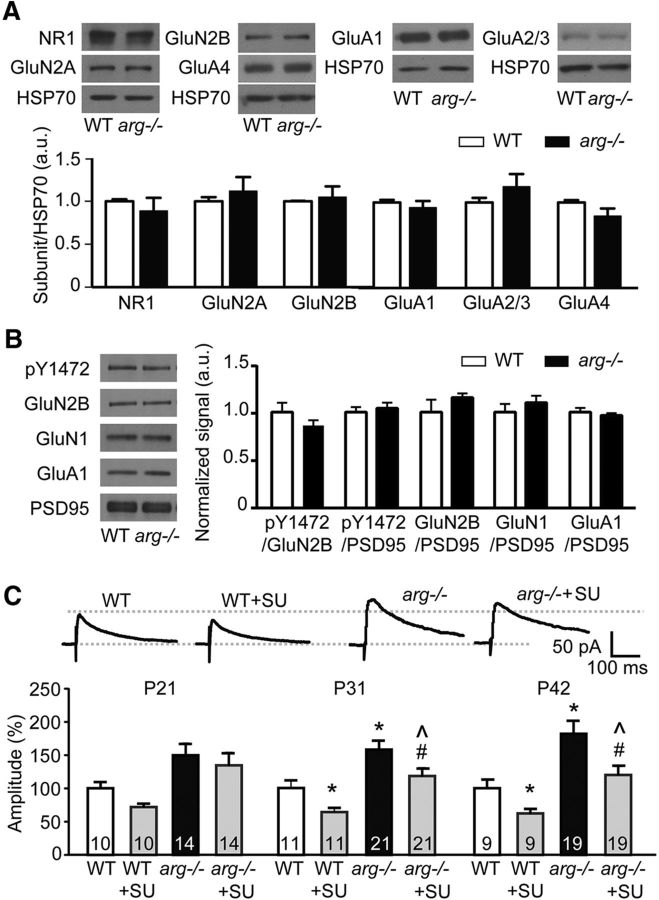
Figure 6.
Src-mediated GluN2B tyrosine phosphorylation does not underlie the increased NMDAR-EPSCs in arg−/− mice. A, Whole-cell hippocampal levels of NMDAR and AMPAR subunits are not changed at P42 in arg−/− mice. Top, Representative Western blots, with HSP70 as a loading control. Bottom, Quantification normalized to HSP70. Protein levels are normalized to WT for each protein combination. B, Tyrosine phosphorylation of Y1472 in the synapse-enriched fraction of the hippocampus is not changed by loss of Arg at P31. In addition, the levels of GluN1, GluN2B, and GluA1 in the synapse-enriched fraction are not changed. Left, Representative Western blots. PSD95 levels are shown as a loading control. Right, Graphed data represent the signal of the first protein normalized to the second, and protein levels are normalized to WT for each protein combination. C, While the Src inhibitor SU6656 (SU) partially blocks NMDAR-EPSCs in both WT and arg−/− neurons at P31 and P42, NMDAR-EPSCs are still increased in arg−/− slices with SU6656 relative to WT slices with SU6656, which differs from the normalization of NMDAR-EPSCs following ifenprodil treatment (Fig. 3). Top, Representative traces of NMDAR-EPSCs at +40 mV in the presence of CNQX from P42 WT and arg−/− mice with or without SU6656. Bottom, Current amplitudes were normalized to the average value calculated from WT at the same age. *p < 0.05, **p < 0.01 vs WT; #p < 0.05 vs arg −/−; ∧p < 0.05 vs WT + SU6656.