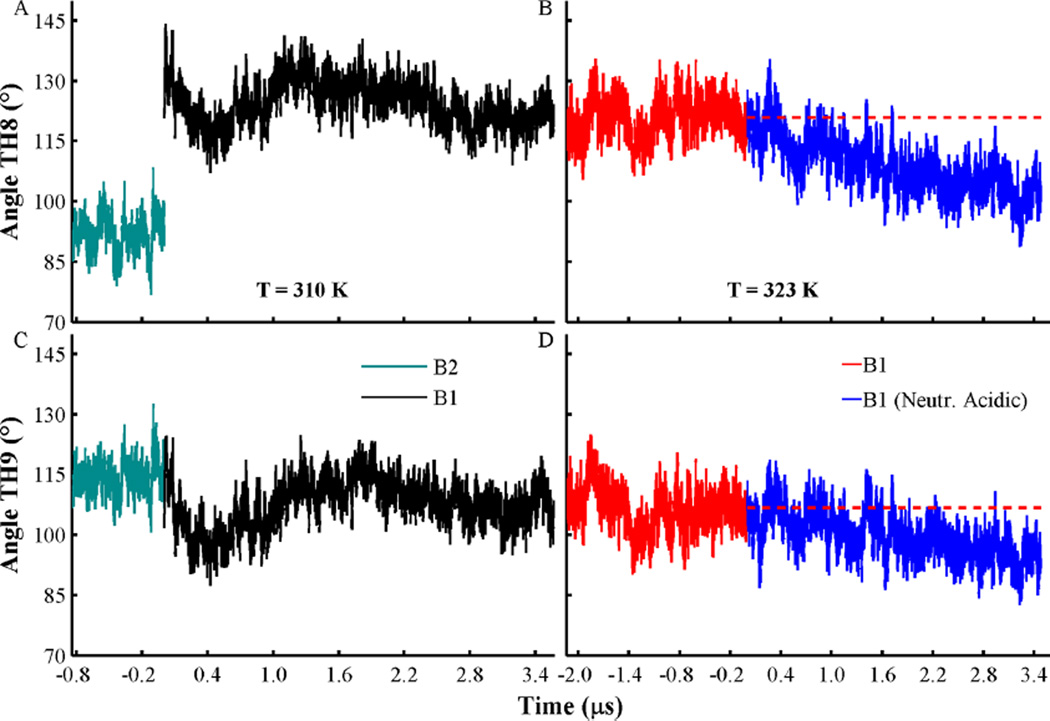
Figure 7.
Changes of the angles formed by the axes of helices TH8 (A, B) and TH9 (C, D) relative to the membrane normal axis. Angle traces obtained from membrane-bound conformations B1 and B2, at T = 310 K, are shown in cyan and black, respectively. Simulations at T = 323 K of membrane-bound conformation B1 with acidic side-chains in their standard and neutralized state are shown in red and blue, respectively. The average values for helices TH8 (121°) and TH9 (107°) obtained from B1 are shown in red broken lines. A value of 90° corresponds to a parallel orientation of a helix relative to the membrane plane. All histidines are set in their protonated state in all simulations, and other acidic residues are set in their standard state, unless indicated.