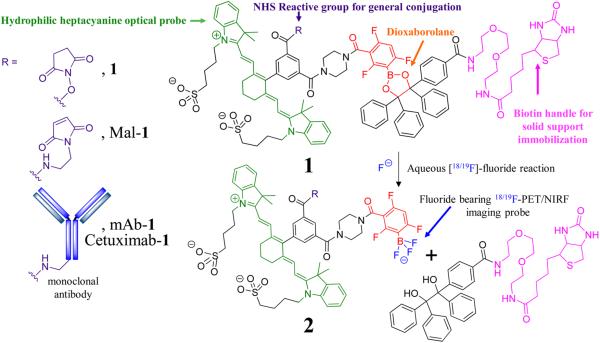
Figure 1.
Chemical structure of the 18F-PET/NIRF probe. The amide reactive 18F-PET/NIRF precursor, 1, the maleimide precursor, Mal-1, and the monoclonal antibody conjugate, mAb-1 (EpCAM) or Cetuximab-1 as R groups (left panel). A fluoride-labile, mAb-1-bearing, solid support is generated when the N-hydroxy succinimide ester (NHS)/maleimide precursor is reacted with mAb and then exposed to a streptavidin bearing support. mAb that is not covalently attached to 1 cannot be retained by the support and is removed with washing. Treatment of the solid support with aqueous fluoride achieves conversion of 1 into a 18/19F labeled trifluoroborate 2, a species useful for PET/NIRF multimodality imaging and simultaneous release of 18F-PET/NIRF labeled mAb-2 from the solid support. Unreacted mAb-1 remains bound to the support through the biotin handle on the solid-phase support (Scheme S1).