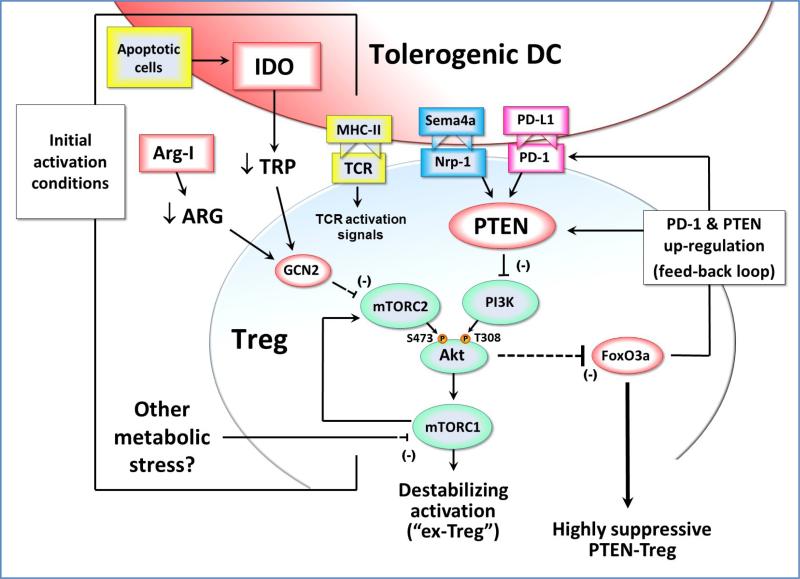
Figure 2. Effects of IDO exposure during Treg activation.
During initial TCR-mediated activation of resting Tregs, signals via the Akt and mTOR pathways can potentially destabilize the Tregs and cause reprogramming into a pro-inflammatory helper-like phenotype (“ex-Tregs”). To prevent this, some signal must inhibit the mTOR/Akt axis during activation. If the antigen-presenting cell expresses IDO (e.g., due to up-regulation by apoptotic cells or other inflammatory signals) then this can inhibit mTORC2 signaling via a process mediated by low tryptophan and GCN2 kinase. In principle, depletion of other amino acids such as arginine (by local Arginase I) could create a similar GCN2-mediated inhibition of mTORC2. Potentially, other metabolic stresses in the tumor microenvironment might likewise affect mTORC1 and the feedback loop to mTORC2 and Akt. By whatever pathway, if Akt phosphorylation on the activating Ser473 residue is blocked, then the Treg is able to maintain expression of FoxO3a and acquire a highly suppressive phenotype that includes up-regulation of cell-surface PD-1 and the lipid phosphatase PTEN. PD-1 can signal via PTEN to inhibit PI3K activity, and thus block phosphorylation of the activating Thr308 residue on Akt. This self-sustaining feedback loop acts to stably maintain the inhibition of Akt long-term, even if the original IDO or other metabolic stress is removed. Neuropilin-1 (Nrp-1) can also activate PTEN, and thus may be able to establish a similar stable activation state in the Treg.