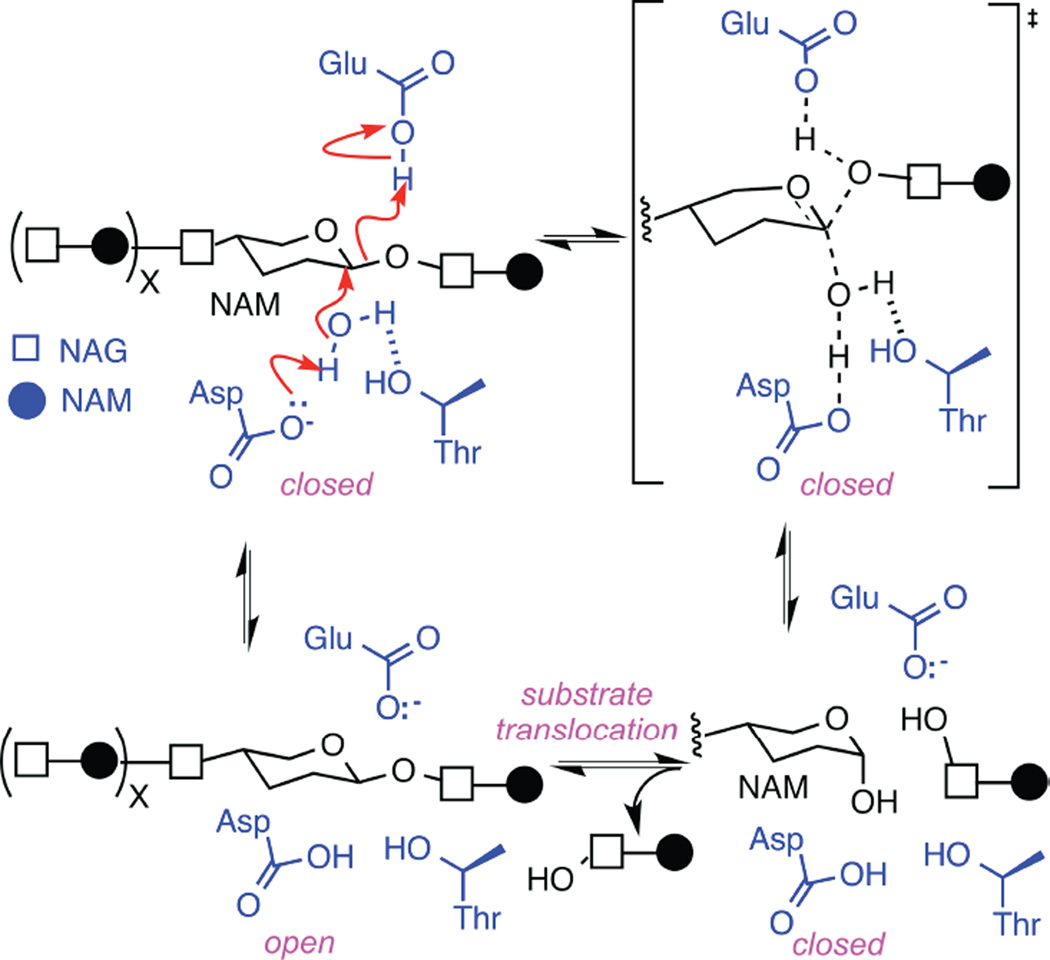
Figure 5.
Hydrolysis of glycosidic bonds catalyzed by T4 lysozyme. The glycosidic bond between N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) subunits are depicted with one water molecule (black) surrounded by lysozyme’s key active-site functionalities Glu11, Asp20, and Thr26 (blue). The concerted mechanism shown with red arrows is observed in 90% of enzyme closures. Interruption of any step leads to the rarer, non-concerted mechanism, and causes the enzyme to pause in an intermediate, higher energy state. As described in the text, identical motions during re-opening suggest that the enzyme remains in the closed conformation during substrate translocation to an identical NAM-NAG glycosidic bond.