Fig. 1.
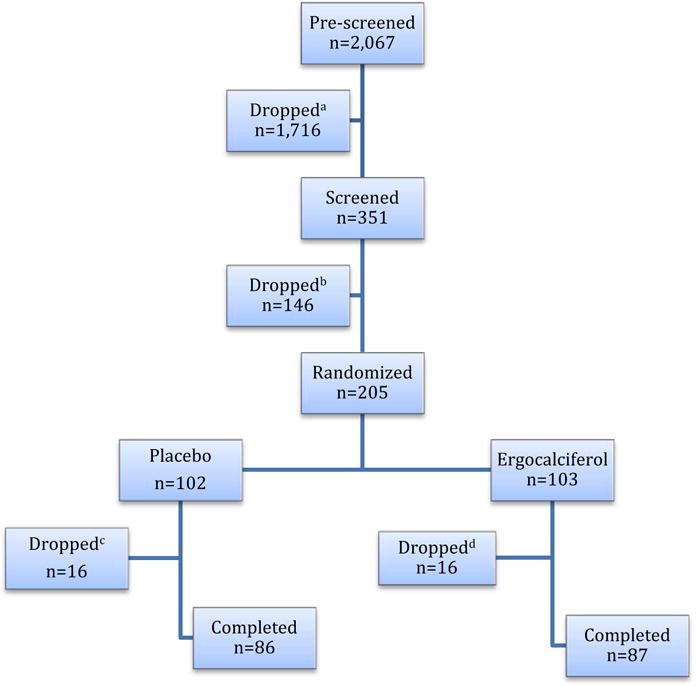
Flow diagram of the trial. aIneligible n=1716: 927 T2DM, 309 BMI<28 or >40 kg/m2, 86 A1C<5.7%, 86 GFR<45 ml/min/1.73m2, 120 on vitamin D supplements, 137 advanced chronic conditions, 51 ineligible sex or race; bDropped n=146: 17 ineligible: 5 A1C<5.7%, 5 A1C>6.9%, 3 GFR<45 ml/min/1.73m2, 4 poor venous access, and 129 lost interest; cDropped n=16: 6 personal issues and lost interest, 2 moved out of state, 2 could not be reached, 6 medical issues (3 depressive disorder and/or substance dependence, 1 hypercalcemia, 1 tumor of the bladder, 1 angioedema due to ACE inhibitor); |dDropped n=16: 4 personal issues and lost interest, 3 moved out of state, 1 could not be reached, 8 medical issues (3 depressive disorder and/or substance dependence, 1 newly diagnosed T2DM requiring medications, 2 liver problems, 1 congestive heart failure, 1 hypokalemia).
