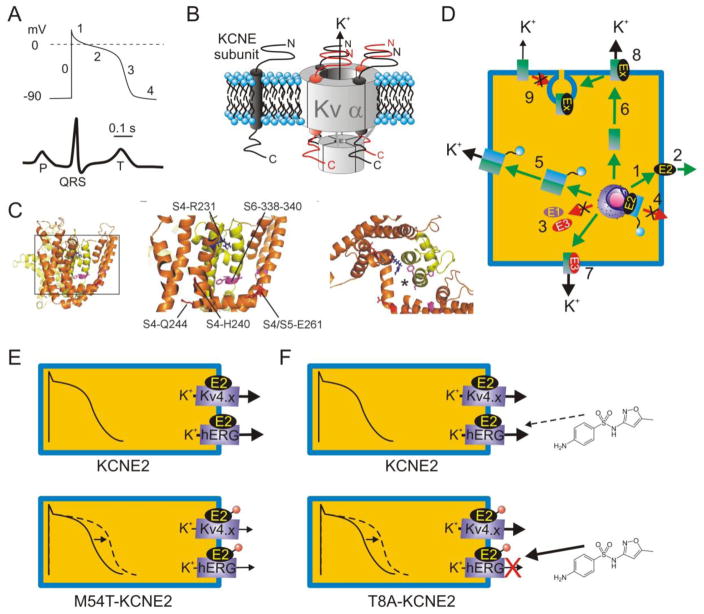
Figure 1.
A. Upper, generic ventricular myocyte action potential indicating phases 0–4; lower, generic surface ECG with time-course and scale-bar corresponding to action potential above.
B. Left, KCNE topology in cell membrane; right, possible stoichiometries of KCNE subunits in complex with a tetramer of Kv α subunits.
C. Putative positioning of KCNE subunits within the Kv channel complex. Left, lateral view of open-state model of KCNQ1 based on Rosetta modeling after the Kv1.2 structure [153–155]. Boxed region is expanded in the center panel, which shows the S6 region known to interact with KCNE subunits and S4/S4–5 linker residues important to KCNQ1 function in the presence and/or absence of KCNE subunits. Right, top view of region in center panel, with asterisk indicating one putative position of KCNE transmembrane region based on structure-function studies.
D. KCNE subunits are extremely versatile, regulating many aspects of K+ channel biology. For example, KCNE2 can reach the surface alone (1) and is also putatively secreted extracellularly (2); KCNE1 and KCNE3 probably require α subunits for surface expression (3). (4) KCNE2 suppresses N-type α subunit surface expression unless they are rescued by same-subfamily non-N-type α subunits (5). (6) KCNE2 and KCNE3 direct contrasting polarized trafficking of α subunits (7), and KCNEs regulate multiple facets of α subunit function (8). KCNE1 and KCNE2 also mediate α subunit endocytosis (9). Ex = KCNE subunit. For detailed review see [80].
E. KCNE2 is thought to form complexes with hERG and Kv4.x channels α subunits in some ventricular cardiomyocytes. Inherited human KCNE2 loss-of-function mutations (red circle) delay myocyte repolarization (dashed line) and are associated with inherited LQTS. One example, KCNE2-M54T, quickens hERG-KCNE2 deactivation [33].
F. The KCNE2-T8A polymorphism is “silent” in the absence of drugs but increases sensitivity to sulfamethoxazole, and can predispose to drug-induced LQTS [99].