Abstract
Background
General practice plays an important role in the cancer care pathway. Patient dissatisfaction with the diagnostic process may be expressed by changing to another general practice.
Aim
To compare the frequency of change of practice (COP) in patients with cancer (n = 150 216) with a matched cancer-free control cohort (n = 1 502 114) and to analyse associations with cancer type and patient characteristics.
Design and setting
A population-based matched cohort study using historical and prospectively collected data from Danish nationwide registers.
Method
COP was defined as a change of practice list, unrelated to change of address or reorganisation of the practice. Data were analysed monthly in the year before and after a cancer diagnosis.
Results
More patients with cancer than controls changed general practice (4.1% versus 2.6%) from 7 months before and until 12 months after diagnosis. The COP rate varied by cancer type (rectal cancer served as reference). Before the diagnosis, COP was most often seen among patients with ovarian cancer (risk ratio [RR] 1.51, 95% confidence interval [CI] = 1.10 to 2.08) and multiple myeloma (RR 1.89, 95% CI = 1.34 to 2.67). After the diagnosis, COP was most frequent among patients with brain cancer (RR 1.38, 95% CI = 1.05 to 1.82) and ovarian cancer (RR 1.51, 95% CI = 1.21 to 1.88).
Conclusion
Patients with cancer changed general practice more frequently than the cancer-free controls. COP variations between cancer types may be attributed to lack of diagnostic timeliness due to clinical complexity of the diagnosis and the role of the GP in the diagnostic process.
Keywords: continuity of patient care, Denmark, early detection of cancer, general practice, neoplasms, primary health care
INTRODUCTION
Cancer is a leading cause of morbidity and mortality in Denmark, as the disease accounts for nearly one in three deaths.1 Timely diagnosis is important and highly prioritised by patients with cancer,2,3 as early detection of cancer may improve the prognosis.4 Most patients with cancer see their GP during the initial diagnostic evaluation and the period after diagnosis.5 Therefore, the GP plays a key role in both diagnosis and follow-up of patients with cancer.6
Referring the right patients for the right diagnostic investigations remains a challenge to GPs, as many cancers, particularly in the early stages, present with vague symptoms.7 Delayed diagnosis may occur if the GP attributes symptoms or abnormal test results to a health problem other than cancer.8 Some patients may then experience a prolonged and dissatisfying diagnostic pathway9 and may express their dissatisfaction through a change of practice (COP).
A COP related to a cancer diagnosis could reflect a poor patient experience of the diagnostic pathway and may jeopardise the integration of primary and specialist care services. Ensuring continuity of care in general practice is highly prioritised, as continuity has been shown to generate better health outcomes,10,11 reduce healthcare costs,12 and strengthen the relationship between patient and GP.13 Patients with cancer changing practice may thus signify a quality problem in general practice, and examining COP may reveal areas for improvement in the diagnostic process in primary care.
This study aimed to compare the frequency of COP among patients with cancer and matched cancer-free controls, to explore if COP is associated with specific cancer types or patient characteristics.
METHOD
Study design
A population-based matched cohort study was performed, using historical, prospectively collected register data, to compare patients with cancer with cancer-free controls. Data were collected from Danish nationwide registers from 1 January 2004 to 31 December 2011 and were linked at the individual level using the unique civil registration number assigned to all Danish citizens at birth or immigration.14
The publicly-funded healthcare system in Denmark provides universal health care to all Danish citizens, and 99% are listed with a specific general practice. The GPs serve as the first point of contact to the healthcare system and gatekeepers to specialised care.15 All citizens must consult their GP for medical advice (except in an emergency), and 85% of all patients with cancer begin the diagnostic pathway in general practice.16
Patients with cancer were identified in the Danish Cancer Register, which holds information on all incident cancer cases in Denmark, including date of diagnosis and type of cancer.17 First-time incident patients with cancer were included (excluding non-melanoma skin cancer, ICD-10: C44), aged ≥18 at date of diagnosis (n = 217 425). Eligible patients must have been living in Denmark from 12 months before until 12 months after diagnosis (n = 216 204; 99%); be listed with a practice 12 months before diagnosis (n = 212 837; 98%); and be alive 12 months after diagnosis (n = 150 216; 69%). Patients who died during the 12 months after diagnosis were excluded, as the intention was to examine quality aspects of continuity among patients with cancer in general practice.
How this fits in
Patients with cancer may express dissatisfaction with the care provided for them by changing to another general practice. Nevertheless, little is known on this issue. These results quantify potential problems in the continuity of care and call for improvements in the clinical diagnostic pathway for some cancer types.
Eligible controls must have been living in Denmark at the index date and be cancer-free until 2 years later. Ten cancer-free controls were matched to each patient with cancer on age, sex, and general practice, by using incidence density sampling. The index date was defined as 12 months before the date of diagnosis of the corresponding cancer patient. Ten eligible controls could not be identified for all patients with cancer (n = 6; 0.004%). If controls emigrated or left the list system, they ceased to contribute to risk time.
Outcome measure
A COP was defined as ‘being listed with another general practice’ if such action was not due to a new postal address (change within 31 days) or restructuring of the general practice (>10% of the patients changed on the same date). Data on COP and practice size were collected from the patient lists, enabling identification of the practice registered for any citizen at any given time; changes between GPs within the same practice are not registered. If more than one COP occurred either before or after diagnosis, only the first on either side of a diagnosis was included.
Study population
Patient addresses were collected from the Danish Civil Registration System.14 The Charlson Comorbidity Index (CCI) was used to assess comorbidity.18,19 The CCI score was calculated by using the diagnoses registered in the Danish National Patient Register 10 years before the index date.20 Only 9 years of data were available for persons with an index date in 2004. Comorbidity was grouped into ‘none’ (CCI score = 0), ‘moderate’ (CCI score = 1–2), and ‘severe’ (CCI score ≥3). Sociodemographic information was collected from Statistics Denmark.21 Data on disposable household income (OECD classification)22 were extracted for the year before the index date, to avoid cancer-related effects on income. Income was grouped into ‘low’, ‘middle’, and ‘high’, based on tertiles. Marital status was grouped into ‘living alone’ or ‘cohabiting’. Educational level was defined in accordance with the International Standard Classification of Education (ISCED)23 and grouped into ‘basic’ (ISCED levels 1–2), ‘short’ (ISCED levels 3–4) and ‘long’ (ISCED levels 5–6). Patients with missing information on educational level were included in the ‘basic’ category (n = 83 959; 4.6%). City size was grouped into ‘rural areas’ (<1000 people); ‘small cities’ (1000–19 999 people); ‘medium cities’ (20 000–99 999 people); and ‘large cities’ (>100 000 people).
Length of GP–patient relationship was defined as time listed with the same practice at the index date and divided into ‘short’ (<5 years), ‘medium’ (5–9 years), and ‘long’ (≥10 years). Based on ICD-10 codes, 23 clinically relevant cancer groups were defined (Appendix 1). Patients who did not fit with any group were classified as having ‘other’ cancers.
Statistical analysis
Analyses were made using binomial regression models24 and presented as risk ratios (RRs) with 95% confidence intervals (CIs). Robust variance estimation was used to account for clustering at practice level or the level of the matched link, where appropriate. The analysis of monthly COP was based on data for 12 months on either side of a diagnosis. The analysis revealed similar RRs for patients with cancer and controls until 7 months before diagnosis. Therefore, the analyses of patient characteristics and cancer types were limited to include only data from 7 months before until 12 months after diagnosis to avoid distortion of estimates.
The analysis of the association between cancer type and COP included only patients with cancer. Rectal cancer was used as reference for comparison of cancer types, as it is a common type of cancer in both sexes.
All analyses were adjusted for age, sex, marital status, educational level, comorbidity, and length of GP–patient relationship. Estimates after diagnosis were additionally adjusted for COP before diagnosis, as it predicted COP after diagnosis. Data were analysed using Stata 13.1.
RESULTS
The study included a total of 150 216 incident patients with cancer and 1 502 114 controls from 2665 general practices. Patients with cancer and controls were similar with respect to patient characteristics (Table 1). A total of 6139 patients with cancer (4.1%) and 39 146 controls (2.6%) changed practice from 7 months before diagnosis until 12 months after.
Table 1.
Characteristics of the 150 216 included Danish patients with cancer and the 1 502 114 matched controls
| Cancer patients | Cancer-free controls | |||
|---|---|---|---|---|
|
|
|
|||
| n | % | n | % | |
| Total | 150 216 | 1 502 114 | ||
| COP 12 months before diagnosis | 3147 | 2.1 | 24 962 | 1.7 |
| COP 12 months after diagnosis | 4020 | 2.7 | 24 334 | 1.6 |
| Total COPa | 7167 | 4.8 | 49 296 | 3.3 |
| Including 7 months before and 12 months after | 6139 | 4.1 | 39 146 | 2.6 |
|
| ||||
| Sex | ||||
| Male | 73 663 | 49.0 | 736 597 | 49.0 |
| Female | 76 553 | 50.9 | 765 517 | 50.9 |
|
| ||||
| Age groups, years | ||||
| 18–44 | 14 217 | 9.5 | 141 717 | 9.4 |
| 45–54 | 19 225 | 12.8 | 191 501 | 12.8 |
| 55–64 | 40 393 | 26.9 | 403 736 | 26.9 |
| 65–74 | 44 069 | 29.3 | 442 322 | 29.5 |
| ≥75 | 32 312 | 21.5 | 322 838 | 21.5 |
|
| ||||
| Charlson Comorbidity Index score | ||||
| None | 119 183 | 79.3 | 1 200 715 | 79.9 |
| Moderate | 26 520 | 17.7 | 255 259 | 16.9 |
| Severe | 4513 | 3.0 | 46 140 | 3.1 |
|
| ||||
| Marital status | ||||
| Cohabiting | 102 472 | 68.2 | 1 016 036 | 67.6 |
| Living alone | 47 739 | 31.8 | 486 053 | 32.4 |
|
| ||||
| Household income | ||||
| Low | 48 735 | 32.4 | 502 041 | 33.4 |
| Middle | 50 695 | 33.8 | 500 082 | 33.3 |
| High | 50 786 | 33.8 | 499 991 | 33.3 |
|
| ||||
| Educational level | ||||
| Basic | 60 906 | 40.6 | 623 053 | 41.5 |
| Short | 57 328 | 38.2 | 562 280 | 37.4 |
| Long | 31 982 | 21.3 | 316 781 | 21.1 |
|
| ||||
| City size | ||||
| Rural area | 32 156 | 21.4 | 328 757 | 21.9 |
| Small city | 50 127 | 33.4 | 495 992 | 33.0 |
| Medium city | 27 558 | 18.4 | 274 922 | 18.3 |
| Large city | 40 375 | 26.9 | 402 443 | 26.8 |
|
| ||||
| Duration of GP–patient relationship, years | ||||
| <5 | 39 445 | 26.3 | 392 588 | 26.1 |
| 5–9 | 25 668 | 17.1 | 256 146 | 17.1 |
| ≥10 | 85 103 | 56.7 | 853 380 | 56.8 |
Including only one COP on either side of diagnosis.
COP = change of practice.
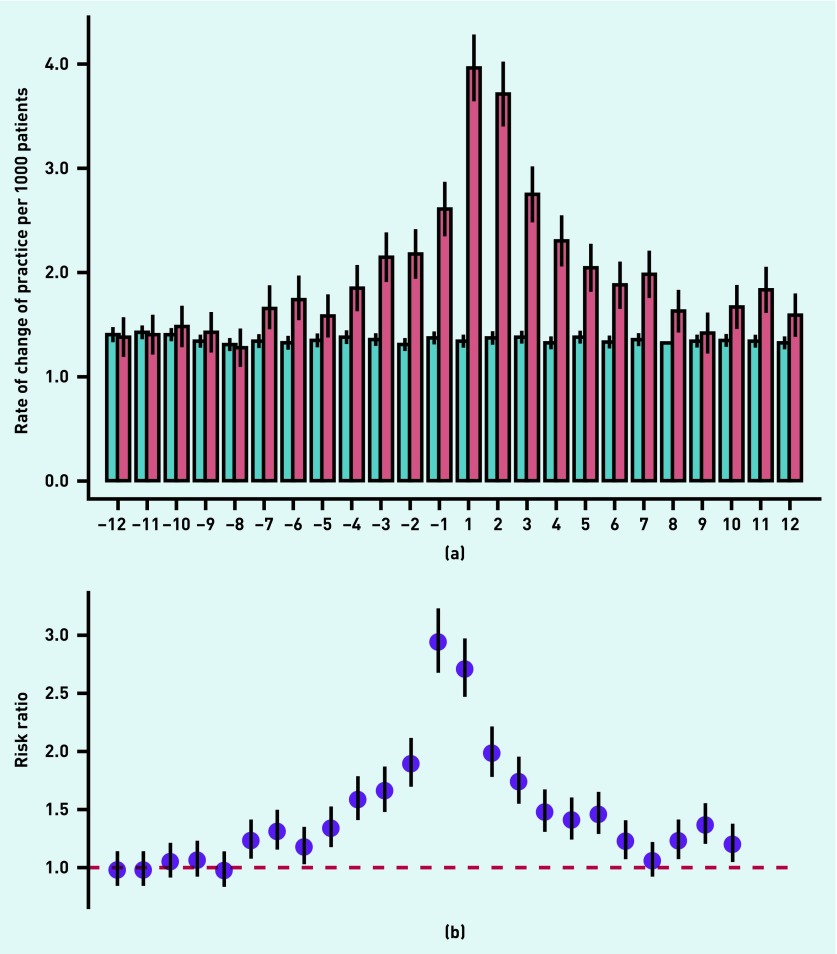
The monthly RR of COP for patients with cancer compared with references began to rise 7 months before diagnosis and increased to 1.88 (95% CI = 1.69 to 2.09) in the month immediately before diagnosis; the RR peaked (RR 2.93; 95% CI = 2.68 to 3.21) in the first month after diagnosis, decreased throughout the year after diagnosis (Figure 1), and levelled with the controls 14 months after diagnosis (data not shown).
Figure 1.
Monthly change of practice (COP), 1 year before and 1 year after the cancer diagnosis compared with matched references. (a) Rates of COP per 1000 patients for patients with cancer (red) and references (blue). (b) Relative risk (RR) for COP among patients with cancer compared with the references. RR was adjusted for age, sex, comorbidity, educational level, marital status, and length of GP–patient relationship.
Before the diagnosis, patients with cancer without comorbidity and a GP–patient relationship >10 years were more likely to change practice compared with other patients with cancer (Table 2). After the diagnosis, patients with cancer aged 45–64 years, without comorbidity, with a high income, with a long education, from rural areas, and with a GP–patient relationship >5 years were more likely to make a COP (Table 2).
Table 2.
The association between change of practice and patient characteristics. Adjusted risk ratio with 95% CI of changing practice from 7 months before diagnosis until 12 months after diagnosis forpatients with cancer compared with the matched references
| COP before diagnosis | COP after diagnosis | |||
|---|---|---|---|---|
|
|
|
|||
| RR | 95% CI | RRa | 95% CI | |
| Sex | ||||
| Female | 1.38 | 1.30 to 1.47 | 1.62 | 1.56 to 1.69 |
| Male | 1.49 | 1.39 to 1.59 | 1.66 | 1.58 to 1.74 |
|
| ||||
| Age groups, years | ||||
| 18–44 | 1.43 | 1.26 to 1.62 | 1.63 | 1.49 to 1.79 |
| 45–54 | 1.44 | 1.27 to 1.62 | 1.86 | 1.71 to 2.03 |
| 55–64 | 1.47 | 1.34 to 1.59 | 1.83 | 1.72 to 1.94 |
| 65–74 | 1.44 | 1.33 to 1.57 | 1.55 | 1.46 to 1.65 |
| >75 | 1.36 | 1.24 to 1.49 | 1.40 | 1.30 to 1.51 |
|
| ||||
| Comorbidity | ||||
| None | 1.46 | 1.39 to 1.53 | 1.72 | 1.66 to 1.78 |
| Moderate | 1.38 | 1.25 to 1.54 | 1.42 | 1.32 to 1.54 |
| Severe | 1.04 | 0.80 to 1.36 | 1.16 | 0.96 to 1.41 |
|
| ||||
| Marital status | ||||
| Cohabiting | 1.43 | 1.35 to 1.51 | 1.67 | 1.60 to 1.74 |
| Living alone | 1.43 | 1.33 to 1.54 | 1.59 | 1.51 to 1.68 |
|
| ||||
| Household income | ||||
| Low | 1.38 | 1.28 to 1.48 | 1.49 | 1.41 to 1.58 |
| Middle | 1.45 | 1.35 to 1.57 | 1.65 | 1.56 to 1.74 |
| High | 1.47 | 1.36 to 1.59 | 1.80 | 1.70 to 1.91 |
|
| ||||
| Educational level | ||||
| Basic | 1.34 | 1.25 to 1.44 | 1.57 | 1.49 to 1.65 |
| Short | 1.54 | 1.43 to 1.65 | 1.62 | 1.54 to 1.71 |
| Long | 1.42 | 1.28 to 1.56 | 1.80 | 1.68 to 1.93 |
|
| ||||
| Duration of GP–patient relationship, years | ||||
| <5 | 1.31 | 1.22 to 1.41 | 1.47 | 1.39 to 1.55 |
| 5–9 | 1.49 | 1.35 to 1.66 | 1.75 | 1.62 to 1.88 |
| ≥10 | 1.53 | 1.42 to 1.64 | 1.77 | 1.69 to 1.86 |
|
| ||||
| City size | ||||
| Rural area | 1.36 | 1.22 to 1.52 | 1.80 | 1.67 to 1.94 |
| Small city | 1.46 | 1.35 to 1.59 | 1.68 | 1.58 to 1.79 |
| Medium city | 1.46 | 1.31 to 1.63 | 1.61 | 1.49 to 1.74 |
| Large city | 1.42 | 1.32 to 1.53 | 1.55 | 1.47 to 1.64 |
Estimates after diagnosis are additionally adjusted for COP before diagnosis. Estimates are adjusted for age, sex, comorbidity, educational level, marital status, and length of GP–patient relationship.
COP = change of practice. RR = risk ratio.
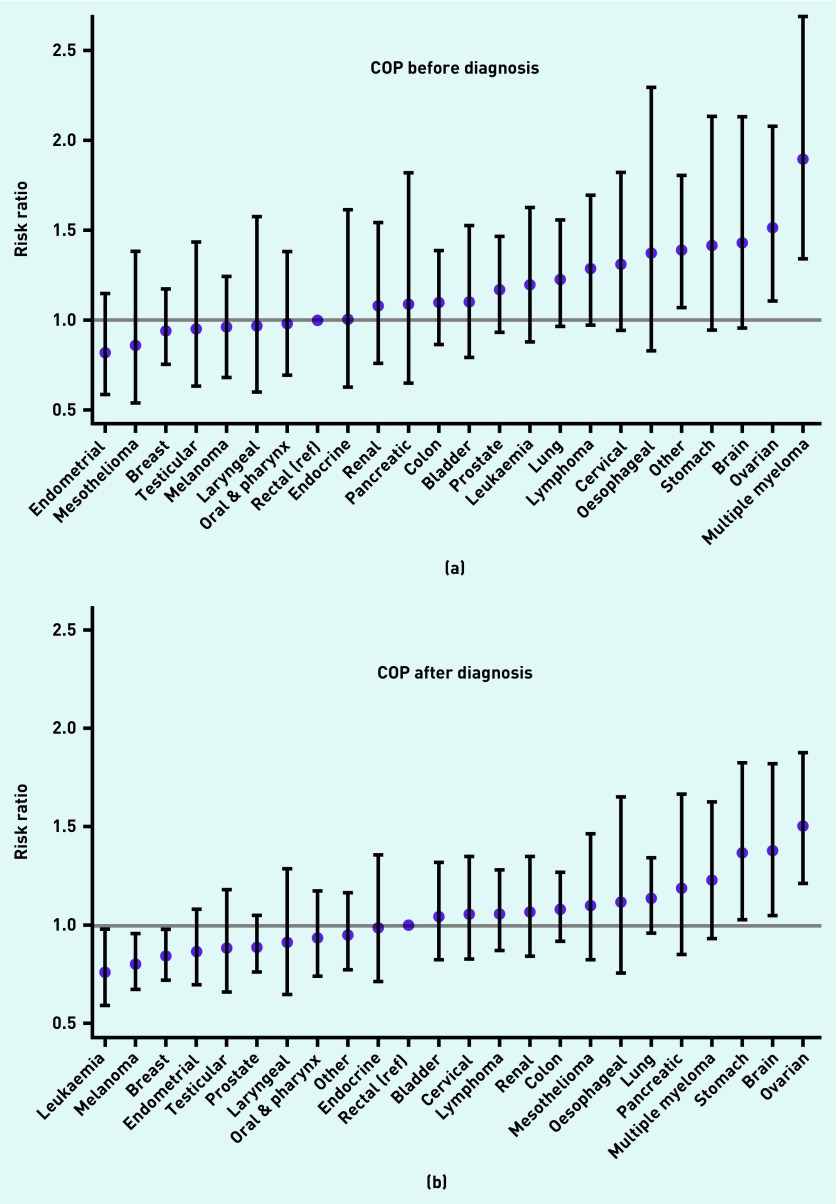
Before the diagnosis, patients with ovarian cancer (RR 1.51; 95% CI = 1.10 to 2.08), multiple myeloma (RR 1.89; 95% CI = 1.34 to 2.67), and other cancers (RR 1.38; 95% CI = 1.07 to 1.80) were more likely to change practice, and patients with breast cancer (RR 0.94; 95% CI = 0.75 to 1.17), mesothelioma (RR 0.86; 95% CI = 0.53 to 1.38), and endometrial cancer (RR 0.82; 95% CI = 0.58 to 1.15) were less likely to change practice compared with patients with rectal cancer (Figure 2). After the diagnosis, patients with stomach cancer (RR 1.37; 95% CI = 1.02 to 1.83), brain cancer (RR 1.38; 95% CI = 1.05 to 1.82), and ovarian cancer (RR 1.51; 95% CI = 1.21 to 1.88) were more likely to change practice, and patients with breast cancer (RR 0.84; 95% CI = 0.72 to 0.98), melanoma (RR 0.79; 95% CI = 0.67 to 0.95), and leukaemia (RR 0.76; 95% CI = 0.59 to 0.98) were less likely to change practice compared with patients with rectal cancer (Figure 2). The RRs for COP were similar for cancer types occurring in both sexes when stratified by sex (data not shown).
Figure 2.
Risk ratios (RRs) of change of practice (COP) by cancer type from 7 months before diagnosis (a) until 12 months after diagnosis (b). Rectal cancer served as the reference. ‘Other’ cancers comprise all cancer types not belonging to the 23 defined cancer groups. RR was adjusted for age, sex, comorbidity, educational level, marital status, and length of GP–patient relationship. Estimates after diagnosis are additionally adjusted for COP before diagnosis.
DISCUSSION
Summary
During a 7-month period before and the year after the diagnosis, Danish patients with cancer changed general practice significantly more than the matched cancer-free controls. Still, the absolute increase in COP was 1.5% points compared with the controls. COP peaked in the month immediately after the diagnosis and was moderated by patient characteristics. The risk of COP varied widely by cancer type.
Strengths and limitations
The study included a nationwide cohort of incident patients with cancer which provided the study with high statistical precision. The nationwide design and the high validity and completeness of the Danish health service registers14,17,20 limited potential bias related to identification or characteristics of patients with cancer.
A limitation was that patients registered with a GP in partnership practices may have changed to another GP within the same practice, and such changes were not registered. The actual rates of COP may thus have been underestimated. Furthermore, this study is limited by the lack of information on reasons for COP, but such information is not routinely collected. However, GP–patient surveys have found ‘overall satisfaction’ to be the strongest predictor of COP.25,26
The date of diagnosis in the Danish Cancer Register corresponds to the earliest time of registration from either hospital (through the Danish National Patient Register) or the Danish Pathology Register.27 This holds a risk of predating the time point when the patient was informed of the cancer, which could have introduced a delay in response time and thus caused a shift in the COP peak towards the right. The date of recorded COP in the Patient List Register generally corresponds to the exact date of COP. However, for a few municipalities, an administrative 14-day delay could have been incurred on the date of COP for new practices located more than 15 km away from the patient’s home. These factors could have contributed to the peak after diagnosis if a COP before diagnosis was misclassified as appearing after diagnosis.
Educational level contained missing information for 83 959 persons (4.6%). These were primarily older persons with no registrations since the establishment of the register. Missing data were distributed equally among patients with cancer and controls, and inclusion was thus not likely to have biased the results.
The risk of confounding was minimised by matching on age, sex, and general practice, noting that patients with cancer and controls exhibited identical risks of COP until 7 months before diagnosis.
Although the CCI does not target diseases managed in general practice and thus may not adjust sufficiently for effects of comorbidity, residual confounding is unlikely to have influenced the conclusions of this study.
Patients who died during the year after diagnosis were excluded. This group changed practice significantly more often, both before and immediately after diagnosis, than did patients surviving at least 12 months (data not shown). Thus, this patient group seems to have a special pattern of COP, and excluding these patients did not increase the risk of COP.
The results of this study are generalisable to countries with similar healthcare systems, where GPs serve as the first point of contact to specialised care.
Comparison with existing literature
Drachmann et al28 found that 3.8% of patients with cancer changed practice during the year after diagnosis: 2.7% among a group representing the general population, and 1.7% among patients with new chronic diseases (other than cancer). In the current study, lower proportions were found among patients with cancer and controls (2.7% and 1.6%) in the year after diagnosis. Nagraj et al25 found even lower COP rates among a general population (1.1%). The differences may be attributed to varying definitions of COP.
To the authors’ knowledge, no studies have investigated COP before receiving a cancer diagnosis. Lyratzopoulos et al29 ranked cancer types according to the risk of having three or more pre-referral consultations with cancer symptoms. The present study’s ranking of cancer types according to risk of COP showed an analogous pattern: ‘easier-to-suspect’ cancers such as melanoma and breast cancer seem to be least likely for COP, and ‘harder-to-suspect’ cancers such as multiple myeloma and ovarian cancer are most likely for COP. This suggests that the risk of COP is strongly influenced by timeliness of the diagnostic process. Patients may experience what they interpret as lack of responsiveness or even GP incompetence. Larsen et al30 found patients with cancer presenting with vague symptoms and experiencing long diagnostic intervals were likely to lose confidence in their GP compared with patients presenting with serious or alarm symptoms, and Mendonca et al31 found patients with three or more pre-referral consultations more likely to report negative experiences of subsequent care.
Patients with a high income, long education, and without comorbidity were more likely candidates for COP after diagnosis. This may indicate that they evaluate their health care more critically. Other patient characteristics revealed no clear patterns with only minor differences between groups.
The access to investigations from general practice may also play a role; direct referral to the necessary diagnostic tests is unavailable to GPs for the cancer types that are most likely of COP and available for the cancer types that are least likely of COP.
Jensen et al32 found patient-reported quality deviations in 29% of cancer diagnostic pathways in general practice. COP was found to be 1.5% points more in the patients with cancer than the matched controls; this suggests that patients tend to stay with their GP even when reporting low-quality treatment.
Implications for practice
Although patients with cancer changed general practice more often than the matched controls, the overall number of COP is low. Lack of continuity of care due to COP in relation to a cancer diagnosis thus seems a minor issue. Still, patients with some cancer types changed practice more frequently, which may indicate that the diagnostic pathways could be optimised. As more patients with cancer changed practice before diagnosis, GPs have the opportunity to capture this.
Acknowledgments
The authors are grateful to Kaare Rud Flarup for assisting in data retrieval from the national registers.
Appendix 1. Cancer types according to the diagnosis codes of the International Classification of Diseases, 10th version (ICD-10)
| Type of cancer | ICD-10 codes |
|---|---|
| Oral & pharynx | C00–C13 |
| Rectal | C20, C21 |
| Oesophageal | C15 |
| Stomach | C16 |
| Colon | C18, C19 |
| Pancreas | C25 |
| Larynx | C32 |
| Lung | C33, C34 |
| Melanoma | C43 |
| Mesothelioma | C45–C49 |
| Breast | C50 |
| Cervix | C53 |
| Endometrial | C54, C55 |
| Ovarian | C56, C570–574 |
| Prostate | C61 |
| Testicular | C62 |
| Renal | C64, C65 |
| Bladder | C67 |
| Brain | C70, C71 |
| Endocrine | C73–C75 |
| Lymphoma | C81–C89 |
| Multiple myeloma | C90 |
| Leukaemia | C91–C96 |
| Other | All other |
Funding
The study was funded by the Danish Cancer Society (reference number A6694).
Ethical approval
Ethical approval was not required according to Danish law, as no identifiable patient information was provided.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
Contributor Information
Kasper Grooss, Research Centre for Cancer Diagnosis in Primary Care, Research Unit for General Practice, Department of Public Health, Aarhus University, Denmark..
Peter Hjertholm, Research Centre for Cancer Diagnosis in Primary Care, Research Unit for General Practice, Department of Public Health, Aarhus University, Denmark..
Anders H Carlsen, Research Centre for Cancer Diagnosis in Primary Care, Research Unit for General Practice, Department of Public Health, Aarhus University, Denmark..
Peter Vedsted, Research Centre for Cancer Diagnosis in Primary Care, Research Unit for General Practice, Department of Public Health, Aarhus University, Denmark..
REFERENCES
- 1.Statens Serum Institute Dødsårsagsregisteret 2013: tal og analyse. [The Danish Registers of causes of death 2013: numbers and analyses]. Summary report. http://www.ssi.dk/dar (accessed 19 Apr 2016)
- 2.Booij JC, Zegers M, Evers PM, et al. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13:203. doi: 10.1186/1471-2407-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101(Suppl 2):S5–S8. doi: 10.1038/sj.bjc.6605383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards MA. The National Awareness and Early Diagnosis Initiative in England: assembling the evidence. Br J Cancer. 2009;101(Suppl 2):S1–S4. doi: 10.1038/sj.bjc.6605382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedsted P, Olesen F. Early diagnosis of cancer: the role of general practice. Scand J Prim Health Care. 2009;27(4):193–194. doi: 10.3109/02813430903478623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. 2009;59(563):441–447. doi: 10.3399/bjgp09X420860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vedsted P, Hansen RP, Bro F. General practice and early cancer diagnosis. [In Danish] Ugeskr Laeger. 2011;173(24):12–15. [PubMed] [Google Scholar]
- 8.Macleod U, Mitchell ED, Burgess C, et al. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(Suppl 2):S92–S101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen H, Nissen A, Vedsted P. Quality deviations in cancer diagnosis: prevalence and time to diagnosis in general practice. Br J Gen Pract. 2014;64(619):92–98. doi: 10.3399/bjgp14X677149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Malley AS, Forrest CB. Beyond the examination room: primary care performance and the patient-physician relationship for low-income women. J Gen Intern Med. 2002;17(1):66–74. doi: 10.1046/j.1525-1497.2002.10338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parchman ML, Burge SK. The patient-physician relationship, primary care attributes, and preventive services. Fam Med. 2004;36(1):22–27. [PubMed] [Google Scholar]
- 12.Gabel LL, Lucas JB, Westbury RC. Why do patients continue to see the same physician? Fam Pract Res J. 1993;13(2):133–147. [PubMed] [Google Scholar]
- 13.Baker R, Mainous AG, 3rd, Gray DP, Love MM. Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors. Scand J Prim Health Care. 2003;21(1):27–32. doi: 10.1080/0283430310000528. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen KM, Andersen JS, Sondergaard J. General practice and primary health care in Denmark. J Am Board Fam Med. 2012;25(1):34–38. doi: 10.3122/jabfm.2012.02.110216. [DOI] [PubMed] [Google Scholar]
- 16.Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(Suppl 7):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson Comorbidity Index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(Suppl 7):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Denmark Documentation of statistics. http://www.dst.dk/en/Statistik/dokumentation/documentationofstatistics (accessed 21 Apr 2016)
- 22.OECD Project on Income Distribution and Poverty What are equivalence scales? http://www.oecd.org/eco/growth/OECD-Note-EquivalenceScales.pdf (accessed 19 Apr 2016)
- 23.UNESCO International standard classification of education ISCED. http://www.uis.unesco.org/Education/Pages/international-standard-classification-of-education.aspx (accessed 19 Apr 2016) [Google Scholar]
- 24.McCullagh P, Nelder JA. Generalized linear models. 2nd edn. London: Chapman & Hall/CRC; 1989. [Google Scholar]
- 25.Nagraj S, Abel G, Paddison C, et al. Changing practice as a quality indicator for primary care: analysis of data on voluntary disenrollment from the English GP Patient Survey. BMC Fam Pract. 2013;14:89. doi: 10.1186/1471-2296-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roblin DW, Roberts MH. Patient dissatisfaction as a determinant of voluntary disenrollment in a managed care organization. J Ambul Care Manage. 2010;33(2):163–172. doi: 10.1097/JAC.0b013e3181d916b2. [DOI] [PubMed] [Google Scholar]
- 27.Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39(7):72–74. doi: 10.1177/1403494810393563. [DOI] [PubMed] [Google Scholar]
- 28.Drachmann TT, Storgaard L, Olesen F. Is change of GP more frequent among patients diagnosed with cancer or other serious diseases? [In Danish] Ugeskr Laeger. 2003;165(27):43–46. [PubMed] [Google Scholar]
- 29.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 30.Larsen MB, Hansen RP, Olesen F, et al. Patients’ confidence in their GP before and after being diagnosed with cancer. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X572409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendonca SC, Abel GA, Saunders CL, et al. Pre-referral general practitioner consultations and subsequent experience of cancer care: evidence from the English Cancer Patient Experience Survey. Eur J Cancer Care. 2015 doi: 10.1111/ecc.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen H, Sperling C, Sandager M, et al. Agreement between patients and general practitioners on quality deviations during the cancer diagnostic pathway and associations with time to diagnosis. Fam Pract. 2015;32(3):329–335. doi: 10.1093/fampra/cmv021. [DOI] [PubMed] [Google Scholar]