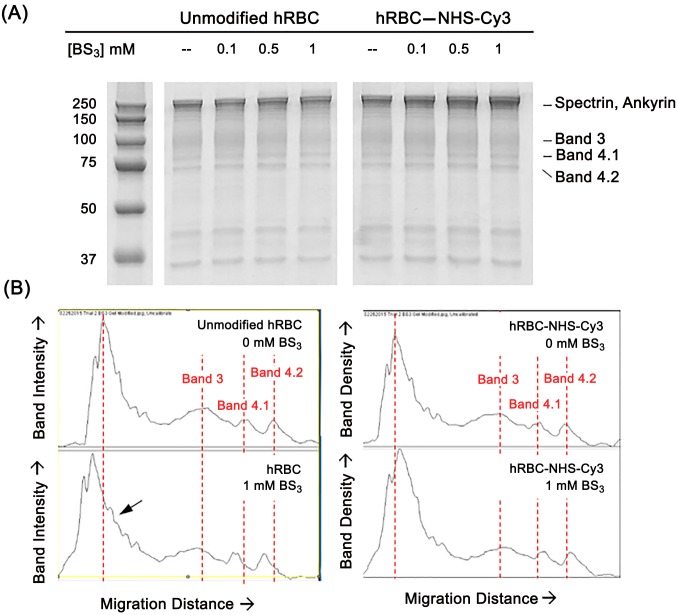
Fig 5. The effect of human erythrocyte membrane engineering with NHS-pDMAA-Cy3 polymers on Band 3 aggregation.
Human erythrocyte Band 3 protein is a major site of NHS-pDMAA-Cy3 modification supported by the absence of Band 3 aggregate formation in the presence of the protein cross-linker bis(sulfosuccinimidyl)suberate (BS3). (A) Isolated hRBC membranes for each group were run on 12% TGX polyacrylamide gels and stained with Imperial protein stain. Gel image truncated after 37 kDa molecular weight marker to highlight larger molecular weight membrane proteins. (B) Histogram plots of band intensity versus migration distance were plotted for each experimental group and concentration (0 mM and 1 mM BS3 concentrations shown). Dotted lines are used to represent peak intensity of specific protein bands in non-crosslinked hRBC membranes. Specifically, at the highest concentration of BS3 (1 mM) there is a noticeable decrease in monomeric Band 3 protein band intensity as well as a modest increase in higher molecular weight proteins gathering at the top of the gel (e.g., in the region of spectrin and ankyrin proteins) for unmodified hRBCs versus those modified with NHS-pDMAA-Cy3 polymers. The arrow represents Band 3 aggregates.